Different Immune Control of Gram-Positive and Gram-Negative Mammary Infections in Dairy Cows
- PMID: 38668433
- PMCID: PMC11054201
- DOI: 10.3390/vetsci11040166
Different Immune Control of Gram-Positive and Gram-Negative Mammary Infections in Dairy Cows
Abstract
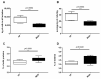
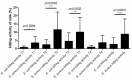
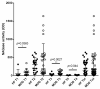
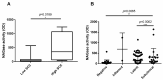
In the dairy industry, bovine mastitis represents a major concern due to substantial production losses and costs related to therapies and early culling. The mechanisms of susceptibility and effective response to intra-mammary infections are still poorly understood. Therefore, we investigated innate immunity in acellular bovine skim milk through cytofluorimetric analyses of bacterial killing activity against both Gram-positive and Gram-negative pathogens. Freshly cultured E. coli and S. aureus strains were incubated with colostrum and milk samples at different lactation time points from two groups of cows, purportedly representing mastitis-resistant and mastitis-susceptible breeds; bacterial cells were analyzed for vitality by flow cytometry following incorporation of vital dyes. N-acetyl-β-D-glucosaminidase (NAGase) activity was also investigated in milk and colostrum samples. Our findings revealed that colostrum and milk bacterial killing activity was greater against S. aureus compared to E. coli., with this activity correlated with milk NAGase levels. Furthermore, both killing of S. aureus and NAGase activity were negatively correlated to the elapsed time of lactation. Interestingly, samples from the allegedly mastitis-resistant breed displayed higher bacterial killing and NAGase activities. Our study suggests that diverse control mechanisms are exerted against Gram-positive and Gram-negative pathogens in the mammary glands of cows, probably beyond those already described in the literature.
Keywords: NAGase; bacterial killing activity; dairy cow; innate immune response; mammary gland; mastitis.
Conflict of interest statement
The authors declare no conflicts of interest. Ferlazzo is now working in a company but at the time of our research, he was working in a research institute (IZSLER). Moreover, all of the research was conducted in research institutes (Unimi and IZSLER) and not in or for a company.
Figures