Perfluorooctanesulfonic Acid Alters Pro-Cancer Phenotypes and Metabolic and Transcriptional Signatures in Testicular Germ Cell Tumors
- PMID: 38668455
- PMCID: PMC11054796
- DOI: 10.3390/toxics12040232
Perfluorooctanesulfonic Acid Alters Pro-Cancer Phenotypes and Metabolic and Transcriptional Signatures in Testicular Germ Cell Tumors
Abstract
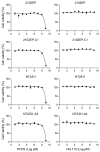
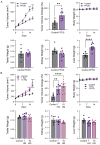
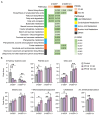
The potential effects of poly- and perfluoroalkyl substances (PFAS) are a recently emergent human and environmental health concern. There is a consistent link between PFAS exposure and cancer, but the mechanisms are poorly understood. Although epidemiological evidence supporting PFAS exposure and cancer in general is conflicting, there is relatively strong evidence linking PFAS and testicular germ cell tumors (TGCTs). However, no mechanistic studies have been performed to date concerning PFAS and TGCTs. In this report, the effects of the legacy PFAS perfluorooctanesulfonic acid (PFOS) and the newer "clean energy" PFAS lithium bis(trifluoromethylsulfonyl)imide (LiTFSi, called HQ-115), on the tumorigenicity of TGCTs in mice, TGCT cell survival, and metabolite production, as well as gene regulation were investigated. In vitro, the proliferation and survival of both chemo-sensitive and -resistant TGCT cells were minimally affected by a wide range of PFOS and HQ-115 concentrations. However, both chemicals promoted the growth of TGCT cells in mouse xenografts at doses consistent with human exposure but induced minimal acute toxicity, as assessed by total body, kidney, and testis weight. PFOS, but not HQ-115, increased liver weight. Transcriptomic alterations of PFOS-exposed normal mouse testes were dominated by cancer-related pathways and gene expression alterations associated with the H3K27me3 polycomb pathway and DNA methylation, epigenetic pathways that were previously showed to be critical for the survival of TGCT cells after cisplatin-based chemotherapy. Similar patterns of PFOS-mediated gene expression occurred in PFOS-exposed cells in vitro. Metabolomic studies revealed that PFOS also altered metabolites associated with steroid biosynthesis and fatty acid metabolism in TGCT cells, consistent with the proposed ability of PFAS to mimic fatty acid-based ligands controlling lipid metabolism and the proposed role of PFAS as endocrine disrupters. Our data, is the first cell and animal based study on PFAS in TGCTs, support a pro-tumorigenic effect of PFAS on TGCT biology and suggests epigenetic, metabolic, and endocrine disruption as potential mechanisms of action that are consistent with the non-mutagenic nature of the PFAS class.
Keywords: GenX; H3K27me3; HQ-115; LiTFSI; PFOS; fatty acid metabolism; metabolomics; steroid synthesis; testicular cancer; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Interstate Technology and Regulatory Council PFAS Technical and Regulatory Guidance Document and Fact Sheets. 2022. [(accessed on 10 December 2022)]. Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2022/09/PFAS-Guidance-Docu....
-
- Organization for Economic Co-Operation and Development Toward a New Comprehensive Global Database oF Per- and Polyfluoroalkyl Substance (PFAS): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFAS) 2018. [(accessed on 10 December 2023)]. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=EN....
-
- Sunderland E.M., Hu X.C., Dassuncao C., Tokranov A.K., Wagner C.C., Allen J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFAS) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019;29:131–147. doi: 10.1038/s41370-018-0094-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

