Results of Response-Guided Therapy with Pegylated Interferon Alpha 2a in Chronic Hepatitis B and D
- PMID: 38668534
- PMCID: PMC11054492
- DOI: 10.3390/tropicalmed9040073
Results of Response-Guided Therapy with Pegylated Interferon Alpha 2a in Chronic Hepatitis B and D
Abstract
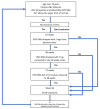
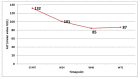
Pegylated interferon alpha 2a continues to be used for the treatment of chronic hepatitis D. The reported on-treatment virologic response varies between 17 and 47%, with relapses in more than 50% of these patients. No stopping rules have been defined, and the duration of the treatment is not clearly established, but it should be between 48 and 96 weeks. In total, 76 patients with compensated liver disease treated with peg-interferon according to the Romanian National protocol for the treatment of hepatitis D were retrospectively included. The duration of treatment was up to 96 weeks, with the following stopping rules: less than a 2 log HDV RNA decrease by week 24 and less than a 1 log decrease every 6 months afterwards. Six months after stopping the treatment, it can be restarted for unlimited cycles. The inclusion criteria were aged above 18, HBs Ag-positive, HDV RNA detectable, ALT above ULN and/or liver fibrosis at least F1 at liver biopsy, or Fibrotest and/or Fibroscan higher than 7 KPa and/or inflammation at least A1 at liver biopsy or Fibrotest. We monitored our patients for a total period of 4 years (including those that repeated the cycle). After the first 6 months of treatment, 27 patients (35.5%) had a greater than 2 log HDV RNA decrease, 19 of them achieving undetectable HDV RNA. Seventeen patients (22.3%) had undetectable HDV RNA 24 weeks after stopping 96 weeks of treatment, and none relapsed in the following 2 years. Of these 17 patients, 6 were cirrhotic, and 4 had F3. Undetectable HDV RNA at 24 weeks was the only parameter that predicted a long-term suppression of HDV RNA. In 49 patients, the treatment was stopped after 6 months according to protocol, but it was restarted 6 months later. Five of these patients finished a 48-week course of treatment; none achieved undetectable HDV RNA. During the first course of therapy, 45 patients had at least one moderate adverse reaction to treatment. In one patient, the treatment was stopped due to a serious adverse event (osteomyelitis). Treatment doses had to be reduced in 29 patients. The virologic response at week 24 can select the patients who will benefit from continuing the treatment from those who should be changed to another type of medication when available.
Keywords: HDV RNA; hepatitis D; pegylated interferon alpha; response guided; treatment; virologic response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

