Divergence of Classical and C-Ring-Cleaved Angucyclines: Elucidation of Early Tailoring Steps in Lugdunomycin and Thioangucycline Biosynthesis
- PMID: 38668630
- PMCID: PMC11106748
- DOI: 10.1021/acschembio.4c00082
Divergence of Classical and C-Ring-Cleaved Angucyclines: Elucidation of Early Tailoring Steps in Lugdunomycin and Thioangucycline Biosynthesis
Abstract
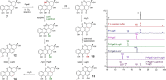
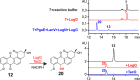
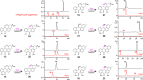
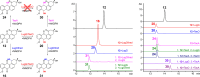
Angucyclines are an important group of microbial natural products that display tremendous chemical diversity. Classical angucyclines are composed of a tetracyclic benz[a]anthracene scaffold with one ring attached at an angular orientation. However, in atypical angucyclines, the polyaromatic aglycone is cleaved at A-, B-, or C-rings, leading to structural rearrangements and enabling further chemical variety. Here, we have elucidated the branching points in angucycline biosynthesis leading toward cleavage of the C-ring in lugdunomycin and thioangucycline biosynthesis. We showed that 12-hydroxylation and 6-ketoreduction of UWM6 are shared steps in classical and C-ring-cleaved angucycline pathways, although the bifunctional 6-ketoreductase LugOIIred harbors additional unique 1-ketoreductase activity. We identified formation of the key intermediate 8-O-methyltetrangomycin by the LugN methyltransferase as the branching point toward C-ring-cleaved angucyclines. The final common step in lugdunomycin and thioangucycline biosynthesis is quinone reduction, catalyzed by the 7-ketoreductases LugG and TacO, respectively. In turn, the committing step toward thioangucyclines is 12-ketoreduction catalyzed by TacA, for which no orthologous protein exists on the lugdunomycin pathway. Our results confirm that quinone reductions are early tailoring steps and, therefore, may be mechanistically important for subsequent C-ring cleavage. Finally, many of the tailoring enzymes harbored broad substrate promiscuity, which we utilized in combinatorial enzymatic syntheses to generate the angucyclines SM 196 A and hydranthomycin. We propose that enzyme promiscuity and the competition of many of the enzymes for the same substrates lead to a branching biosynthetic network and formation of numerous shunt products typical for angucyclines rather than a canonical linear metabolic pathway.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Weber S.; Zolke C.; Rohr J.; Beale J. M. Investigations of the Biosynthesis and Structural Revision of Landomycin A. J. Org. Chem. 1994, 59 (15), 4211–4214. 10.1021/jo00094a037. - DOI
-
- Hoffmeister D.; Ichinose K.; Domann S.; Faust B.; Trefzer A.; Dräger G.; Kirschning A.; Fischer C.; Künzel E.; Bearden D. W.; Rohr J.; Bechthold A. The NDP-Sugar Co-Substrate Concentration and the Enzyme Expression Level Influence the Substrate Specificity of Glycosyltransferases: Cloning and Characterization of Deoxysugar Biosynthetic Genes of the Urdamycin Biosynthetic Gene Cluster. Chem. Biol. 2000, 7 (11), 821–831. 10.1016/S1074-5521(00)00029-6. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

