Anisotropic Liesegang pattern for the nonlinear elastic biomineral-hydrogel complex
- PMID: 38669324
- PMCID: PMC11051667
- DOI: 10.1126/sciadv.adl3075
Anisotropic Liesegang pattern for the nonlinear elastic biomineral-hydrogel complex
Abstract
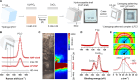
The Liesegang pattern is a beautiful natural anisotropic patterning phenomenon observed in rocks and sandstones. This study reveals that the Liesegang pattern can induce nonlinear elasticity. Here, a Liesegang-patterned complex with biomineral-hydrogel repetitive layers is prepared. This Liesegang-patterned complex is obtained only when the biomineralization is performed under the supersaturated conditions. The Liesegang-patterned complex features a nonlinear elastic response, whereas a complex with a single biomineral shell shows a linear behavior, thus demonstrating that the Liesegang pattern is essential in achieving nonlinear elasticity. The stiff biomineral layers have buffered the concentrated energy on behalf of soft hydrogels, thereby exposing the hydrogel components to reduced stress and, in turn, enabling them to perform the elasticity continuously. Moreover, the nonlinear elastic Liesegang-patterned complex exhibits excellent stress relaxation to the external loading, which is the biomechanical characteristic of cartilage. This stress relaxation allows the bundle of fiber-type Liesegang-patterned complex to endure greater deformation.
Figures





References
-
- Guimarães C. F., Gasperini L., Marques A. P., Reis R. L., The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
-
- Quan H., Kisailus D., Meyers M. A., Hydration-induced reversible deformation of biological materials. Nat. Rev. Mater. 6, 264–283 (2021).
-
- Hua M., Wu S., Ma Y., Zhao Y., Chen Z., Frenkel I., Strzalka J., Zhou H., Zhu X., He X., Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599 (2021). - PubMed
LinkOut - more resources
Full Text Sources

