Vagus nerve stimulation modulates distinct acetylcholine receptors on B cells and limits the germinal center response
- PMID: 38669336
- PMCID: PMC11051663
- DOI: 10.1126/sciadv.adn3760
Vagus nerve stimulation modulates distinct acetylcholine receptors on B cells and limits the germinal center response
Abstract
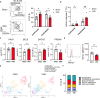
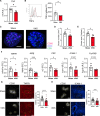
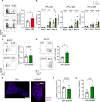
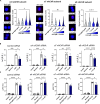
Acetylcholine is produced in the spleen in response to vagus nerve activation; however, the effects on antibody production have been largely unexplored. Here, we use a chronic vagus nerve stimulation (VNS) mouse model to study the effect of VNS on T-dependent B cell responses. We observed lower titers of high-affinity IgG and fewer antigen-specific germinal center (GC) B cells. GC B cells from chronic VNS mice exhibited altered mRNA and protein expression suggesting increased apoptosis and impaired plasma cell differentiation. Follicular dendritic cell (FDC) cluster dispersal and altered gene expression suggested poor function. The absence of acetylcholine-producing CD4+ T cells diminished these alterations. In vitro studies revealed that α7 and α9 nicotinic acetylcholine receptors (nAChRs) directly regulated B cell production of TNF, a cytokine crucial to FDC clustering. α4 nAChR inhibited coligation of CD19 to the B cell receptor, presumably decreasing B cell survival. Thus, VNS-induced GC impairment can be attributed to distinct effects of nAChRs on B cells.
Figures


References
-
- Bonaz B., Sinniger V., Hoffmann D., Clarençon D., Mathieu N., Dantzer C., Vercueil L., Picq C., Trocmé C., Faure P., Cracowski J.-L., Pellissier S., Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterol. Motil. 28, 948–953 (2016). - PubMed
-
- Koopman F. A., Chavan S. S., Miljko S., Grazio S., Sokolovic S., Schuurman P. R., Mehta A. D., Levine Y. A., Faltys M., Zitnik R., Tracey K. J., Tak P. P., Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in Rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 113, 8284–8289 (2016). - PMC - PubMed
-
- Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J., Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003). - PubMed
-
- Meroni E., Stakenborg N., Gomez-Pinilla P. J., Stakenborg M., Aguilera-Lizarraga J., Florens M., Delfini M., de Simone V., De Hertogh G., Goverse G., Matteoli G., Boeckxstaens G. E., Vagus nerve stimulation promotes epithelial proliferation and controls colon monocyte infiltration During DSS-induced colitis. Front. Med. 8, 694268 (2021). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

