Modified Delphi panel consensus recommendations for management of severe aplastic anemia
- PMID: 38669341
- PMCID: PMC11331724
- DOI: 10.1182/bloodadvances.2023011642
Modified Delphi panel consensus recommendations for management of severe aplastic anemia
Abstract
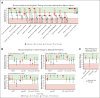
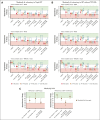
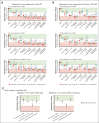
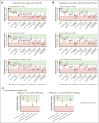
Severe aplastic anemia (SAA) is a rare hematologic condition for which there is no clear management algorithm. A panel of 11 experts on adult and pediatric aplastic anemia was assembled and, using the RAND/University of California, Los Angeles modified Delphi panel method, evaluated >600 varying patient care scenarios to develop clinical recommendations for the initial and subsequent management of patients of all ages with SAA. Here, we present the panel's recommendations to rule out inherited bone marrow failure syndromes, on supportive care before and during first-line therapy, and on first-line (initial management) and second-line (subsequent management) therapy of acquired SAA, focusing on when transplant vs medical therapy is most appropriate. These recommendations represent the consensus of 11 experts informed by published literature and experience. They are intended only as general guidance for experienced clinicians who treat patients with SAA and are in no way intended to supersede individual physician and patient decision making. Current and future research should validate this consensus using clinical data. Once validated, we hope these expert panel recommendations will improve outcomes for patients with SAA.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors have completed International Committee of Medical Journal Editors Disclosure of Interest forms. D.V.B. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports research grants from the National Institutes of Health and the American Society of Hematology (ASH); reports payment or honoraria for participation in Highlights of ASH from the American Society of Hematology; and reports stock or stock options from Carisma Inc. A.E.D. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Geron, Regeneron, Sobi, Caribou, Apellis, Bristol Myers Squibb, and Novartis. C.D. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Novartis for a steering committee; reports payment or honoraria from Alexion and Apellis; and reports participation on a data safety monitoring board or advisory board from Regeneron. Z.R.R. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports honoraria for lecture on Bone Marrow Failure for Sub-specialty Board Review Course (2021) from the American Society of Pediatric Hematology/Oncology; reports payment or honoraria from UpToDate as author of Schwachman Diamond Topic Card; reports payment or honoraria from WebMD (formerly Medscape) as author of Pearson’s Syndrome Topic; reports participation on a data safety monitoring board or advisory board as a panel member for the Federal Data Safety Monitoring Board (DSMB #1) for the National Institutes of Health/the National Heart, Lung, and Blood Institute Bone Marrow Transplant Clinical Trials Network; reports leadership as past chair, with review of AAP hematology-related issues unpaid for the American Academy of Pediatrics Section on Hematology Oncology executive committee; and reports other financial or nonfinancial interests for programmatic review/grant review panel member as the chair from 2023 from the Bone Marrow Failure Research Program of the Congressionally Mandated Research Program. D.B. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. M.S.B. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. S.F. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Genentech and Incyte; reports payment or honoraria from Takeda, Bristol Myers Squibb, and Sanofi; reports participation on a data safety monitoring board or advisory board from Genmab and AbbVie; and reports leadership or fiduciary role in other board, society, committee, or advocacy group paid or unpaid from South Carolina Oncology Society. S.N.G. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. R.H. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis. J.P.M. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports grants or contracts from National Institutes of Health, Leukemia and Lymphoma Society, and US Department of Defense; reports consulting fees for participation on an advisory board from Regeneron and Novartis, and consulting fees for MOS Registry from Bristol Myers Squibb, and consulting fees for PNH Registry from Alexion Pharmaceuticals; reports payment or honoraria for an online debate from Novartis; and reports participation on a data safety monitoring board or advisory board from Omeros. B.S. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Bristol Myers Squibb, Alexion, Novartis, and Incyte; reports payment or honoraria from Bristol Myers Squibb, Alexion, Novartis, and Jazz; reports participation on a data safety monitoring board or advisory board from Nektar, Alexion, and Bristol Myers Squibb; and reports leadership or fiduciary role in other board, society, committee, or advocacy group paid or unpaid from ASH Government Affairs. S.T. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports grants or contracts from Karyopharm Therapeutics Inc as an investigator who initiated clinical trial and preclinical studies; reports consulting fees from consulting/advisory board from Karyopharm Therapeutics Inc, Novartis, AbbVie, MorphoSys, and CTI Biopharma; and reports support for attending meetings and/or travel from Karyopharm Therapeutics for 2023 AACR meeting. M.W.W. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis. I.Y. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. B.J.P. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis and to help write the manuscript, participation as the panel chair, being an active panelist and working on the manuscript with Novartis (note this was a blinded sponsored project, and payment was made to B.J.P.); and reports payment for training and education on Promacta with Novartis (payment was made to B.J.P.).
Figures

References
-
- Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia - PubMed. Haematologica. 2008;93(4):489–492. - PubMed
-
- Zhang J, Liu T, Duan Y, et al. Single-cell analysis highlights a population of Th17-polarized CD4+ naïve T cells showing IL6/JAK3/STAT3 activation in pediatric severe aplastic anemia. J Autoimmun. 2023;136 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

