Myosin-1C augments endothelial secretion of von Willebrand factor by linking contractile actomyosin machinery to the plasma membrane
- PMID: 38669344
- PMCID: PMC11413703
- DOI: 10.1182/bloodadvances.2024012590
Myosin-1C augments endothelial secretion of von Willebrand factor by linking contractile actomyosin machinery to the plasma membrane
Abstract
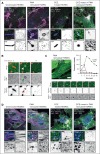
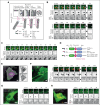
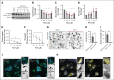
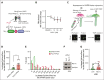
Blood endothelial cells control the hemostatic and inflammatory response by secreting von Willebrand factor (VWF) and P-selectin from storage organelles called Weibel-Palade bodies (WPBs). Actin-associated motor proteins regulate this secretory pathway at multiple points. Before fusion, myosin Va forms a complex that anchors WPBs to peripheral actin structures, allowing for the maturation of content. After fusion, an actomyosin ring/coat is recruited and compresses the WPB to forcibly expel the largest VWF multimers. Here, we provide, to our knowledge, the first evidence for the involvement of class I myosins during regulated VWF secretion. We show that the unconventional myosin-1C (Myo1c) is recruited after fusion via its pleckstrin homology domain in an actin-independent process. This provides a link between the actin ring and phosphatidylinositol 4,5-bisphosphate (PIP2) at the membrane of the fused organelle and is necessary to ensure maximal VWF secretion. This is an active process requiring Myo1c ATPase activity because inhibition of class I myosins using the inhibitor pentachloropseudilin or expression of an ATPase-deficient Myo1c rigor mutant perturbs the expulsion of VWF and alters the kinetics of the exocytic actin ring. These data offer a novel insight into the control of an essential physiological process and provide a new way in which it can be regulated.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
Myo1c drives actin-dependent VWF expulsion from EC WPBs.Blood Adv. 2024 Sep 10;8(17):4711-4713. doi: 10.1182/bloodadvances.2024013476. Blood Adv. 2024. PMID: 39254967 Free PMC article. No abstract available.
References
-
- Larsen E, Celi A, Gilbert GE, et al. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59(2):305–312. - PubMed
-
- McCormack JJ, Lopes da Silva M, Ferraro F, Patella F, Cutler DF. Weibel−Palade bodies at a glance. J Cell Sci. 2017;130(21):3611–3617. - PubMed
-
- Tsai HM, Nagel RL, Hatcher VB, Seaton AC, Sussman II. The high molecular weight form of endothelial cell von Willebrand factor is released by the regulated pathway. Br J Haematol. 1991;79(2):239–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

