Scaling-Up Microwave-Assisted Synthesis of Highly Defective Pd@UiO-66-NH2 Catalysts for Selective Olefin Hydrogenation under Ambient Conditions
- PMID: 38669483
- PMCID: PMC11082845
- DOI: 10.1021/acsami.4c03106
Scaling-Up Microwave-Assisted Synthesis of Highly Defective Pd@UiO-66-NH2 Catalysts for Selective Olefin Hydrogenation under Ambient Conditions
Abstract
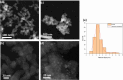
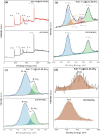
The need to develop green and cost-effective industrial catalytic processes has led to growing interest in preparing more robust, efficient, and selective heterogeneous catalysts at a large scale. In this regard, microwave-assisted synthesis is a fast method for fabricating heterogeneous catalysts (including metal oxides, zeolites, metal-organic frameworks, and supported metal nanoparticles) with enhanced catalytic properties, enabling synthesis scale-up. Herein, the synthesis of nanosized UiO-66-NH2 was optimized via a microwave-assisted hydrothermal method to obtain defective matrices essential for the stabilization of metal nanoparticles, promoting catalytically active sites for hydrogenation reactions (760 kg·m-3·day-1 space time yield, STY). Then, this protocol was scaled up in a multimodal microwave reactor, reaching 86% yield (ca. 1 g, 1450 kg·m-3·day-1 STY) in only 30 min. Afterward, Pd nanoparticles were formed in situ decorating the nanoMOF by an effective and fast microwave-assisted hydrothermal method, resulting in the formation of Pd@UiO-66-NH2 composites. Both the localization and oxidation states of Pd nanoparticles (NPs) in the MOF were achieved using high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and X-ray photoelectron spectroscopy (XPS), respectively. The optimal composite, loaded with 1.7 wt % Pd, exhibited an extraordinary catalytic activity (>95% yield, 100% selectivity) under mild conditions (1 bar H2, 25 °C, 1 h reaction time), not only in the selective hydrogenation of a variety of single alkenes (1-hexene, 1-octene, 1-tridecene, cyclohexene, and tetraphenyl ethylene) but also in the conversion of a complex mixture of alkenes (i.e., 1-hexene, 1-tridecene, and anethole). The results showed a powerful interaction and synergy between the active phase (Pd NPs) and the catalytic porous scaffold (UiO-66-NH2), which are essential for the selectivity and recyclability.
Keywords: UiO-66-NH2; defect engineering; gram-scale; microwave-assisted synthesis; palladium; selective hydrogenation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- de Vries J. G.; Jackson S. D. Homogeneous and Heterogeneous Catalysis in Industry. Catal. Sci. Technol. 2012, 2 (10), 200910.1039/c2cy90039d. - DOI
-
- Hayler J. D.; Leahy D. K.; Simmons E. M. A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38 (1), 36–46. 10.1021/acs.organomet.8b00566. - DOI
LinkOut - more resources
Full Text Sources

