Separators and Membranes for Advanced Alkaline Water Electrolysis
- PMID: 38669641
- PMCID: PMC11117188
- DOI: 10.1021/acs.chemrev.3c00694
Separators and Membranes for Advanced Alkaline Water Electrolysis
Abstract
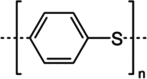
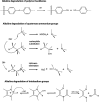
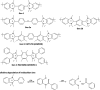
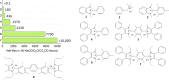
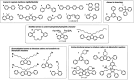
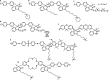
Traditionally, alkaline water electrolysis (AWE) uses diaphragms to separate anode and cathode and is operated with 5-7 M KOH feed solutions. The ban of asbestos diaphragms led to the development of polymeric diaphragms, which are now the state of the art material. A promising alternative is the ion solvating membrane. Recent developments show that high conductivities can also be obtained in 1 M KOH. A third technology is based on anion exchange membranes (AEM); because these systems use 0-1 M KOH feed solutions to balance the trade-off between conductivity and the AEM's lifetime in alkaline environment, it makes sense to treat them separately as AEM WE. However, the lifetime of AEM increased strongly over the last 10 years, and some electrode-related issues like oxidation of the ionomer binder at the anode can be mitigated by using KOH feed solutions. Therefore, AWE and AEM WE may get more similar in the future, and this review focuses on the developments in polymeric diaphragms, ion solvating membranes, and AEM.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






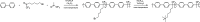
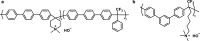
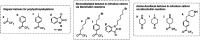











References
-
- https://unfccc.int/process-and-meetings/the-paris-agreement (accessed 2023-07-27).
-
- Modisha P.; Bessarabov D. Aromatic Liquid Organic Hydrogen Carriers for Hydrogen Storage and Release. Curr. Opin. Green Sustain. Chem. 2023, 42, 100820. 10.1016/j.cogsc.2023.100820. - DOI
-
- Niermann M.; Beckendorff A.; Kaltschmitt M.; Bonhoff K. Liquid Organic Hydrogen Carrier (Lohc) - Assessment Based on Chemical and Economic properties. Int. J. Hydrog. Energy 2019, 44, 6631–6654. 10.1016/j.ijhydene.2019.01.199. - DOI
-
- Chen Y. Global Potential of Algae-Based Photobiological Hydrogen Production. Energy Environ. Sci. 2022, 15, 2843–2857. 10.1039/D2EE00342B. - DOI
Publication types
LinkOut - more resources
Full Text Sources

