Watch & wait - Post neoadjuvant imaging for rectal cancer
- PMID: 38669916
- PMCID: PMC11090716
- DOI: 10.1016/j.clinimag.2024.110166
Watch & wait - Post neoadjuvant imaging for rectal cancer
Abstract
Rectal cancer management has evolved over the past decade with the emergence of total neoadjuvant therapy (TNT). For select patients who achieve a clinical complete response following TNT, organ preservation by means of the watch-and-wait (WW) strategy is an increasingly adopted alternative that preserves rectal function and quality of life without compromising oncologic outcomes. Recently, published 5-year results from the OPRA trial demonstrated that organ preservation can be achieved in approximately half of patients managed with the WW strategy, with most local regrowth events occurring within two years. Considering the potential for local regrowth, the implementation of the WW strategy mandates rigorous clinical and radiographic surveillance. Magnetic resonance imaging (MRI) serves as the conventional imaging modality for local staging and surveillance of rectal cancer given its excellent soft-tissue resolution. This review will discuss the current evidence for the WW strategy and the role of restaging rectal MRI in determining patient eligibility for this strategy. Restaging rectal MRI acquisition parameters and treatment response assessment, including important factors to assess, pitfalls, and classification systems, will be discussed in the context of the WW strategy.
Keywords: Magnetic resonance imaging; Neoadjuvant therapy; Rectal cancer; Watch and wait.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest J.J.S. received travel support for fellow education from Intuitive Surgical (August 2015). He also served as a clinical advisor for Guardant Health (March 2019) and as a clinical advisor for Foundation Medicine (April 2022). He served as a consultant and speaker for Johnson and Johnson (May 2022). And he serves as a clinical advisor and consultant for GlaxoSmithKline (2023-24). The remaining authors disclose no conflict of interest.
Figures
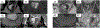



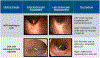
References
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73(3):233–54. - PubMed
-
- Conroy T, Bosset JF, Etienne PL, Rio E, Francois E, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22(5):702–15. - PubMed
-
- Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22(1):29–42. - PubMed
-
- Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2022;40(23):2546–56. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

