Principles and therapeutic applications of adaptive immunity
- PMID: 38670065
- PMCID: PMC11177542
- DOI: 10.1016/j.cell.2024.03.037
Principles and therapeutic applications of adaptive immunity
Abstract
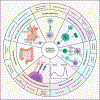
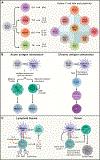
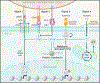
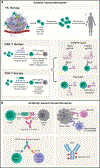
Adaptive immunity provides protection against infectious and malignant diseases. These effects are mediated by lymphocytes that sense and respond with targeted precision to perturbations induced by pathogens and tissue damage. Here, we review key principles underlying adaptive immunity orchestrated by distinct T cell and B cell populations and their extensions to disease therapies. We discuss the intracellular and intercellular processes shaping antigen specificity and recognition in immune activation and lymphocyte functions in mediating effector and memory responses. We also describe how lymphocytes balance protective immunity against autoimmunity and immunopathology, including during immune tolerance, response to chronic antigen stimulation, and adaptation to non-lymphoid tissues in coordinating tissue immunity and homeostasis. Finally, we discuss extracellular signals and cell-intrinsic programs underpinning adaptive immunity and conclude by summarizing key advances in vaccination and engineering adaptive immune responses for therapeutic interventions. A deeper understanding of these principles holds promise for uncovering new means to improve human health.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.C. has consulted for Kumquat Biosciences, Inc., Chugai Pharmaceutical Co., and ONO Pharmaceutical Co. and is a co-inventor on patents/patent applications in the fields of immunotherapy. M.P. serves on the scientific advisory board of Vaxart, Inc. P.G.T. has consulted or received honoraria and/or travel support from Illumina, J&J, Pfizer, Merck, and 10x Genomics, and his lab has received funding from ElevateBio. P.G.T. serves on the scientific advisory boards of ImmunoScape, Shennon Bio, and CytoAgents.
Figures


References
Publication types
MeSH terms
Grants and funding
- R01 AI150514/AI/NIAID NIH HHS/United States
- 75N93021C00016/AI/NIAID NIH HHS/United States
- R01 AI176545/AI/NIAID NIH HHS/United States
- R01 CA265009/CA/NCI NIH HHS/United States
- R37 AI105887/AI/NIAID NIH HHS/United States
- U01 AI144616/AI/NIAID NIH HHS/United States
- U01 CA281868/CA/NCI NIH HHS/United States
- R56 AI177874/AI/NIAID NIH HHS/United States
- R01 AI136514/AI/NIAID NIH HHS/United States
- R35 CA253188/CA/NCI NIH HHS/United States
- R01 AI150241/AI/NIAID NIH HHS/United States
- 75N93021C00018/AI/NIAID NIH HHS/United States
- R01 AI131703/AI/NIAID NIH HHS/United States
- R01 AI154470/AI/NIAID NIH HHS/United States
- R01 AI177874/AI/NIAID NIH HHS/United States
- U01 AI165457/AI/NIAID NIH HHS/United States
- U01 AI150747/AI/NIAID NIH HHS/United States
- R01 AI140761/AI/NIAID NIH HHS/United States
- R01 AI105887/AI/NIAID NIH HHS/United States
- 75N93019C00052/AI/NIAID NIH HHS/United States
- U01 AI142001/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

