Development of a chimeric cytokine receptor that captures IL-6 and enhances the antitumor response of CAR-T cells
- PMID: 38670095
- PMCID: PMC11148643
- DOI: 10.1016/j.xcrm.2024.101526
Development of a chimeric cytokine receptor that captures IL-6 and enhances the antitumor response of CAR-T cells
Abstract
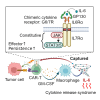
The efficacy of chimeric antigen receptor (CAR)-engineered T cell therapy is suboptimal in most cancers, necessitating further improvement in their therapeutic actions. However, enhancing antitumor T cell response inevitably confers an increased risk of cytokine release syndrome associated with monocyte-derived interleukin-6 (IL-6). Thus, an approach to simultaneously enhance therapeutic efficacy and safety is warranted. Here, we develop a chimeric cytokine receptor composed of the extracellular domains of GP130 and IL6RA linked to the transmembrane and cytoplasmic domain of IL-7R mutant that constitutively activates the JAK-STAT pathway (G6/7R or G6/7R-M452L). CAR-T cells with G6/7R efficiently absorb and degrade monocyte-derived IL-6 in vitro. The G6/7R-expressing CAR-T cells show superior expansion and persistence in vivo, resulting in durable antitumor response in both liquid and solid tumor mouse models. Our strategy can be widely applicable to CAR-T cell therapy to enhance its efficacy and safety, irrespective of the target antigen.
Keywords: CAR-T; IL-6; IL-7; JAK-STAT signaling; adoptive immunotherapy; chimeric antigen receptor; cytokine release syndrome; hematological malignancy; neurotoxicity; solid tumor.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests This study was partly supported by a commercial research grant from Takara Bio, Inc. S.O. and Y.A. are employees of Takara Bio Inc. Aichi Prefecture has filed a patent application related to this study on which Y.K. and T.Y. are named as inventors.
Figures




References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/s1470-2045(18)30864-7. - DOI - PMC - PubMed
-
- Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

