Behavioral changes in patients with Prader-Willi syndrome receiving diazoxide choline extended-release tablets compared to the PATH for PWS natural history study
- PMID: 38671361
- PMCID: PMC11046911
- DOI: 10.1186/s11689-024-09536-x
Behavioral changes in patients with Prader-Willi syndrome receiving diazoxide choline extended-release tablets compared to the PATH for PWS natural history study
Abstract
Background: Prader-Willi syndrome (PWS) is a rare neurobehavioral-metabolic disease caused by the lack of paternally expressed genes in the chromosome 15q11-q13 region, characterized by hypotonia, neurocognitive problems, behavioral difficulties, endocrinopathies, and hyperphagia resulting in severe obesity if energy intake is not controlled. Diazoxide choline extended-release (DCCR) tablets have previously been evaluated for their effects on hyperphagia and other behavioral complications of people with PWS in a Phase 3 placebo-controlled study of participants with PWS, age 4 and older with hyperphagia (C601) and in an open label extension study, C602.
Methods: To better understand the longer-term impact of DCCR, a cohort from PATH for PWS, a natural history study that enrolled participants with PWS age 5 and older, who met the C601 age, weight and baseline hyperphagia inclusion criteria and had 2 hyperphagia assessments ≥ 6 months apart, were compared to the C601/C602 cohort. Hyperphagia was measured using the Hyperphagia Questionnaire for Clinical Trials (HQ-CT, range 0-36). The primary analysis used observed values with no explicit imputation of missing data. A sensitivity analysis was conducted in which all missing HQ-CT assessments in the C601/C602 cohort were assigned the highest possible value (36), representing the worst-case scenario. Other behavioral changes were assessed using the Prader-Willi Syndrome Profile questionnaire (PWSP).
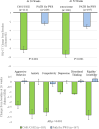
Results: Relative to the PATH for PWS natural history study cohort, the DCCR-treated C601/C602 cohort showed significant improvements in HQ-CT score at 26 weeks (LSmean [SE] -8.3 [0.75] vs. -2.5 [0.43], p < 0.001) and 52 weeks (LSmean [SE] -9.2 [0.77] vs. -3.4 [0.47], p < 0.001). The comparison between the cohorts remained significant in the worst-case imputation sensitivity analysis. There were also significant improvements in all domains of the PWSP at 26 weeks (all p < 0.001) and 52 weeks (all p ≤ 0.003) for C601/C602 participants compared to the PATH for PWS participants.
Conclusion: Long-term administration of DCCR to people with PWS resulted in changes in hyperphagia and other behavioral complications of PWS that are distinct from the natural history of the syndrome as exemplified by the cohort from PATH for PWS. The combined effects of administration of DCCR should reduce the burden of the syndrome on the patient, caregivers and their families, and thereby may benefit people with PWS and their families.
Trial registration: Clinical study C601 was originally registered on ClinicalTrials.gov on February 22, 2018 (NCT03440814). Clinical study C602 was originally registered on ClinicalTrials.gov on October 22, 2018 (NCT03714373). PATH for PWS was originally registered on ClinicalTrials.gov on October 24, 2018 (NCT03718416).
Keywords: DCCR; Hyperphagia; Natural history; Prader-Willi syndrome.
© 2024. The Author(s).
Conflict of interest statement
SB, PH, KY, NC and AB are employed by Soleno Therapeutics. The institution of JLM, SEM, EG, and JAY received funding from Soleno to support the conduct of the clinical trial. EG reports receipt of lecturing and consulting fees from Soleno. JAY reports grant support from Soleno Therapeutics and from Rhythm Pharmaceuticals for obesity-related projects, and material support for research from Hikma Pharmaceuticals and Versanis-Bio and is supported by the Intramural Research Program of the
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous