DEC1 is involved in circadian rhythm disruption-exacerbated pulmonary fibrosis
- PMID: 38671456
- PMCID: PMC11046974
- DOI: 10.1186/s12964-024-01614-w
DEC1 is involved in circadian rhythm disruption-exacerbated pulmonary fibrosis
Abstract
Background: The alveolar epithelial type II cell (AT2) and its senescence play a pivotal role in alveolar damage and pulmonary fibrosis. Cell circadian rhythm is strongly associated with cell senescence. Differentiated embryonic chondrocyte expressed gene 1 (DEC1) is a very important circadian clock gene. However, the role of DEC1 in AT2 senescence and pulmonary fibrosis was still unclear.
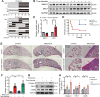
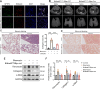
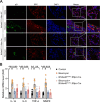
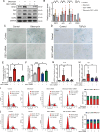
Results: In this study, a circadian disruption model of light intervention was used. It was found that circadian disruption exacerbated pulmonary fibrosis in mice. To understand the underlying mechanism, DEC1 levels were investigated. Results showed that DEC1 levels increased in lung tissues of IPF patients and in bleomycin-induced mouse fibrotic lungs. In vitro study revealed that bleomycin and TGF-β1 increased the expressions of DEC1, collagen-I, and fibronectin in AT2 cells. Inhibition of DEC1 mitigated bleomycin-induced fibrotic changes in vitro and in vivo. After that, cell senescence was observed in bleomycin-treated AT2 cells and mouse models, but these were prevented by DEC1 inhibition. At last, p21 was confirmed having circadian rhythm followed DEC1 in normal conditions. But bleomycin disrupted the circadian rhythm and increased DEC1 which promoted p21 expression, increased p21 mediated AT2 senescence and pulmonary fibrosis.
Conclusions: Taken together, circadian clock protein DEC1 mediated pulmonary fibrosis via p21 and cell senescence in alveolar epithelial type II cells.
Keywords: Alveolar epithelial type II cell; DEC1; Pulmonary fibrosis; p21.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures





References
-
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205:e18–47. doi: 10.1164/rccm.202202-0399ST. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. 82200081/the National Natural Science Foundation of China
- No. 82070098/the National Natural Science Foundation of China
- No. 82270021/the National Natural Science Foundation of China
- No. 82270075 and 82070066/the National Natural Science Foundation of China
- No. 82270111 and 81973991/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

