Recent advances in the discovery and development of drugs targeting the kallikrein-kinin system
- PMID: 38671481
- PMCID: PMC11046790
- DOI: 10.1186/s12967-024-05216-5
Recent advances in the discovery and development of drugs targeting the kallikrein-kinin system
Abstract
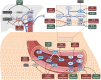
Background: The kallikrein-kinin system is a key regulatory cascade involved in blood pressure maintenance, hemostasis, inflammation and renal function. Currently, approved drugs remain limited to the rare disease hereditary angioedema. However, growing interest in this system is indicated by an increasing number of promising drug candidates for further indications.
Methods: To provide an overview of current drug development, a two-stage literature search was conducted between March and December 2023 to identify drug candidates with targets in the kallikrein-kinin system. First, drug candidates were identified using PubMed and Clinicaltrials.gov. Second, the latest publications/results for these compounds were searched in PubMed, Clinicaltrials.gov and Google Scholar. The findings were categorized by target, stage of development, and intended indication.
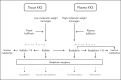
Results: The search identified 68 drugs, of which 10 are approved, 25 are in clinical development, and 33 in preclinical development. The three most studied indications included diabetic retinopathy, thromboprophylaxis and hereditary angioedema. The latter is still an indication for most of the drug candidates close to regulatory approval (3 out of 4). For the emerging indications, promising new drug candidates in clinical development are ixodes ricinus-contact phase inhibitor for thromboprophylaxis and RZ402 and THR-149 for the treatment of diabetic macular edema (all phase 2).
Conclusion: The therapeutic impact of targeting the kallikrein-kinin system is no longer limited to the treatment of hereditary angioedema. Ongoing research on other diseases demonstrates the potential of therapeutic interventions targeting the kallikrein-kinin system and will provide further treatment options for patients in the future.
Keywords: B1 receptor; B2 receptor; Bradykinin; Coagulation factor XII; Diabetic macular edema; Diabetic retinopathy; Kallikrein-kinin system; Plasma kallikrein; Thromboprophylaxis; Tissue kallikrein.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

