Shining a spotlight on the inclusion of disabled participants in clinical trials: a mixed methods study
- PMID: 38671497
- PMCID: PMC11046956
- DOI: 10.1186/s13063-024-08108-7
Shining a spotlight on the inclusion of disabled participants in clinical trials: a mixed methods study
Abstract
Background: It is crucial to include a wide range of the population in clinical trials for the outcome to be applicable in real-world settings. Existing literature indicates that under-served groups, including disabled people, have been excluded from participating in clinical trials without justification. Exclusion from clinical trials exacerbates disparities in healthcare and diminishes the benefits for excluded populations. Therefore, this study was conducted to investigate potential obstacles that prevent disabled people from participating in clinical trials in the United Kingdom (UK).
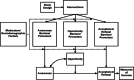
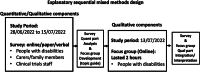
Methods: The study was carried out through an explanatory sequential mixed methods design. The Imperial Clinical Trials Unit devised and implemented an online questionnaire-based survey (with open/closed-ended questions) and an online focus group discussion. The target population were disabled people, family members/carers of disabled people and staff involved in clinical trials, whereupon the sample was recruited by convenience sampling methods via posters and emails through various networks. The Qualtrics XM survey system was used as the host platform for the online survey, and Microsoft Teams was used for an online focus group discussion. The focus group discussion was conducted to gain a deeper understanding of the themes identified from the survey responses. We analysed responses to the survey via descriptive analysis and used thematic analysis to synthesise the free-text answers from the survey and focus group discussion.
Results: We received 45 responses to the survey questionnaire and 5 disabled people took part in a focus group discussion. Our findings highlighted the differences between the perspectives of researchers and those "being researched" and different types of barriers experienced by disabled people: opportunity barriers (inadequate recruitment strategy and ambiguous eligibility criteria), awareness barriers (perception of disability) and acceptance/refusal barriers (available support and adjustment, and sharing of trial results).
Conclusion: Our findings support perspectives drawn from the Ford Framework regarding the need to consider all barriers, not just up to the point of enrolment into trials but also beyond the point of inclusion in clinical trials. We support calls for the introduction of legislation on including disabled people in clinical trials, implementation of industry/community-wide participatory approaches and the development of guidelines, a combined public-private approach.
Keywords: Carers; Clinical trials; Disability; Disabled people; Diversity; Equality; Exclusion; Inclusion; Mixed methods study; Under-served groups.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. Clinical trials. 2024. https://www.who.int/health-topics/clinical-trials#tab=tab_1. Accessed 9 Mar 2024.
-
- Larson E. Exclusion of certain groups from clinical research. Image--the journal of nursing scholarship. 1994;26(3):185–190. 10.1111/j.1547-5069.1994.tb00311.x. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical