Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study
- PMID: 38671808
- PMCID: PMC11048622
- DOI: 10.3390/bioengineering11040387
Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study
Abstract
Aim: The objective of this in vitro study was to compare reused and sterilized versus new healing abutments to assess whether a decontamination and sterilization process performed on resued healing abutments was sufficient to remove residual proteins. The two groups were comparable with respect to patient safety.
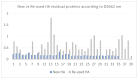
Materials and methods: During the period from September 2022 to October 2023, healing abutment screws were selected and divided into two groups according to whether they were new or previously used in patients. The samples were subjected to a decontamination and sterilization protocol, and results from sample sterility evaluation and assessment of surface protein levels were recorded.
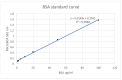
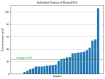
Results: The obtained results revealed a significant difference in the OD562 nm values between new and reused healing abutment samples. The assay demonstrates how treated healing abutments were still contaminated by residual proteins.
Conclusion: Within the limitations of the present study, although from an infectious point of view sterilization results in the total eradication of pathogens, surface proteins remain on reused healing abutments.
Keywords: contamination; dental disinfectants; dental implant; healing abutment; sterilization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Evaluation of residual contamination on reused healing abutments.Clin Oral Investig. 2021 Oct;25(10):5889-5895. doi: 10.1007/s00784-021-03894-9. Epub 2021 Mar 25. Clin Oral Investig. 2021. PMID: 33763713
-
Is the re-use of sterilized implant abutments safe enough? (Implant abutment safety).Med Oral Patol Oral Cir Bucal. 2019 Sep 1;24(5):e583-e587. doi: 10.4317/medoral.22967. Med Oral Patol Oral Cir Bucal. 2019. PMID: 31433387 Free PMC article.
-
Is it safe to reuse dental implant healing abutments sterilized and serviced by dealers of dental implant manufacturers? An in vitro sterility analysis.Implant Dent. 2015 Apr;24(2):174-9. doi: 10.1097/ID.0000000000000198. Implant Dent. 2015. PMID: 25706262
-
Should Healing Abutments and Cover Screws for Dental Implants be Reused? A Systematic Review.J Prosthodont. 2020 Jan;29(1):42-48. doi: 10.1111/jopr.13106. Epub 2019 Sep 16. J Prosthodont. 2020. PMID: 31453645
-
Dental implant healing abutment decontamination: A systematic review of in vitro studies.Int J Oral Implantol (Berl). 2022 Nov 15;15(4):311-324. Int J Oral Implantol (Berl). 2022. PMID: 36377623
Cited by
-
A comparison of four decontamination procedures in Reusing healing abutments: An in vitro study.Saudi Dent J. 2024 Aug;36(8):1141-1145. doi: 10.1016/j.sdentj.2024.06.013. Epub 2024 Jun 14. Saudi Dent J. 2024. PMID: 39176159 Free PMC article.
-
Green Dentistry: State of the Art and Possible Development Proposals.Dent J (Basel). 2025 Jan 16;13(1):38. doi: 10.3390/dj13010038. Dent J (Basel). 2025. PMID: 39851612 Free PMC article. Review.
References
-
- Fernandes D., Nunes S., López-Castro G., Marques T., Montero J., Borges T. Effect of customized healing abutments on the peri-implant linear and volumetric tissue changes at maxillary immediate implant sites: A 1-year prospective randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2021;23:745–757. doi: 10.1111/cid.13044. - DOI - PubMed
-
- Kyaw T.T., Abdou A., Nakata H., Pimkhaokham A. Dental implant healing abutment decontamination: A systematic review of in vitro studies. Int. J. Oral Implantol. 2022;15:311–324. - PubMed
LinkOut - more resources
Full Text Sources

