Effects of L-Type Voltage-Gated Calcium Channel (LTCC) Inhibition on Hippocampal Neuronal Death after Pilocarpine-Induced Seizure
- PMID: 38671837
- PMCID: PMC11047745
- DOI: 10.3390/antiox13040389
Effects of L-Type Voltage-Gated Calcium Channel (LTCC) Inhibition on Hippocampal Neuronal Death after Pilocarpine-Induced Seizure
Abstract
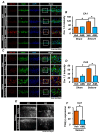
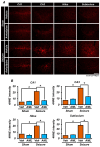
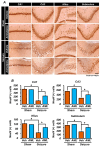
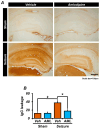
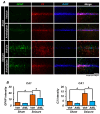
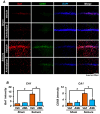
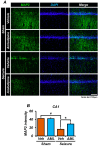
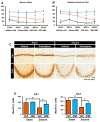
Epilepsy, marked by abnormal and excessive brain neuronal activity, is linked to the activation of L-type voltage-gated calcium channels (LTCCs) in neuronal membranes. LTCCs facilitate the entry of calcium (Ca2+) and other metal ions, such as zinc (Zn2+) and magnesium (Mg2+), into the cytosol. This Ca2+ influx at the presynaptic terminal triggers the release of Zn2+ and glutamate to the postsynaptic terminal. Zn2+ is then transported to the postsynaptic neuron via LTCCs. The resulting Zn2+ accumulation in neurons significantly increases the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits, contributing to reactive oxygen species (ROS) generation and neuronal death. Amlodipine (AML), typically used for hypertension and coronary artery disease, works by inhibiting LTCCs. We explored whether AML could mitigate Zn2+ translocation and accumulation in neurons, potentially offering protection against seizure-induced hippocampal neuronal death. We tested this by establishing a rat epilepsy model with pilocarpine and administering AML (10 mg/kg, orally, daily for 7 days) post-epilepsy onset. We assessed cognitive function through behavioral tests and conducted histological analyses for Zn2+ accumulation, oxidative stress, and neuronal death. Our findings show that AML's LTCC inhibition decreased excessive Zn2+ accumulation, reactive oxygen species (ROS) production, and hippocampal neuronal death following seizures. These results suggest amlodipine's potential as a therapeutic agent in seizure management and mitigating seizures' detrimental effects.
Keywords: L-type voltage-gated calcium channel; amlodipine; neuronal death; oxidative stress; seizure; zinc.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Raised activity of L-type calcium channels renders neurons prone to form paroxysmal depolarization shifts.Neuromolecular Med. 2013 Sep;15(3):476-92. doi: 10.1007/s12017-013-8234-1. Epub 2013 May 22. Neuromolecular Med. 2013. PMID: 23695859 Free PMC article.
-
Clustering of CaV 1.3 L-type calcium channels by Shank3.J Neurochem. 2023 Oct;167(1):16-37. doi: 10.1111/jnc.15880. Epub 2023 Jun 30. J Neurochem. 2023. PMID: 37392026 Free PMC article.
-
The Transient Receptor Potential Melastatin 7 (TRPM7) Inhibitors Suppress Seizure-Induced Neuron Death by Inhibiting Zinc Neurotoxicity.Int J Mol Sci. 2020 Oct 24;21(21):7897. doi: 10.3390/ijms21217897. Int J Mol Sci. 2020. PMID: 33114331 Free PMC article.
-
Post-treatment of an NADPH oxidase inhibitor prevents seizure-induced neuronal death.Brain Res. 2013 Mar 7;1499:163-72. doi: 10.1016/j.brainres.2013.01.007. Epub 2013 Jan 10. Brain Res. 2013. PMID: 23313582
-
Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury.Curr Mol Med. 2004 Mar;4(2):87-111. doi: 10.2174/1566524043479211. Curr Mol Med. 2004. PMID: 15032707 Review.
Cited by
-
Imipramine, an Acid Sphingomyelinase Inhibitor, Promotes Newborn Neuron Survival in the Hippocampus After Seizure.Cells. 2025 Feb 14;14(4):281. doi: 10.3390/cells14040281. Cells. 2025. PMID: 39996753 Free PMC article.
-
Modulatory Impact of Oxidative Stress on Action Potentials in Pathophysiological States: A Comprehensive Review.Antioxidants (Basel). 2024 Sep 26;13(10):1172. doi: 10.3390/antiox13101172. Antioxidants (Basel). 2024. PMID: 39456426 Free PMC article. Review.
-
Ion Channels in Odor Information Processing of Neural Circuits of the Vertebrate Olfactory Bulb.Int J Mol Sci. 2024 Dec 10;25(24):13259. doi: 10.3390/ijms252413259. Int J Mol Sci. 2024. PMID: 39769024 Free PMC article. Review.
-
Electrophysiological Mechanisms and Therapeutic Potential of Calcium Channels in Atrial Fibrillation.Rev Cardiovasc Med. 2025 Jun 25;26(6):33507. doi: 10.31083/RCM33507. eCollection 2025 Jun. Rev Cardiovasc Med. 2025. PMID: 40630446 Free PMC article. Review.
References
-
- Arnold S.T., Dodson W.E. Epilepsy in children. Baillieres Clin. Neurol. 1996;5:783–802. - PubMed
-
- Jeong J.H., Lee S.H., Kho A.R., Hong D.K., Kang D.H., Kang B.S., Park M.K., Choi B.Y., Choi H.C., Lim M.S., et al. The Transient Receptor Potential Melastatin 7 (TRPM7) Inhibitors Suppress Seizure-Induced Neuron Death by Inhibiting Zinc Neurotoxicity. Int. J. Mol. Sci. 2020;21:7897. doi: 10.3390/ijms21217897. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

