Endothelial NOX5 Obliterates the Reno-Protective Effect of Nox4 Deletion by Promoting Renal Fibrosis via Activation of EMT and ROS-Sensitive Pathways in Diabetes
- PMID: 38671844
- PMCID: PMC11047703
- DOI: 10.3390/antiox13040396
Endothelial NOX5 Obliterates the Reno-Protective Effect of Nox4 Deletion by Promoting Renal Fibrosis via Activation of EMT and ROS-Sensitive Pathways in Diabetes
Abstract
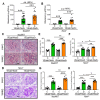
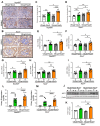
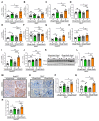
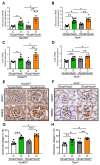
Chronic hyperglycemia induces intrarenal oxidative stress due to the excessive production of reactive oxygen species (ROS), leading to a cascade of events that contribute to the development and progression of diabetic kidney disease (DKD). NOX5, a pro-oxidant NADPH oxidase isoform, has been identified as a significant contributor to renal ROS in humans. Elevated levels of renal ROS contribute to endothelial cell dysfunction and associated inflammation, causing increased endothelial permeability, which can disrupt the renal ecosystem, leading to progressive albuminuria and renal fibrosis in DKD. This study specifically examines the contribution of endothelial cell-specific human NOX5 expression in renal pathology in a transgenic mouse model of DKD. This study additionally compares NOX5 with the previously characterized NADPH oxidase, NOX4, in terms of their relative roles in DKD. Regardless of NOX4 pathway, this study found that endothelial cell-specific expression of NOX5 exacerbates renal injury, albuminuria and fibrosis. This is attributed to the activation of the endothelial mesenchymal transition (EMT) pathway via enhanced ROS formation and the modulation of redox-sensitive factors. These findings underscore the potential therapeutic significance of NOX5 inhibition in human DKD. The study proposes that inhibiting NOX5 could be a promising approach for mitigating the progression of DKD and strengthens the case for the development of NOX5-specific inhibitors as a potential therapeutic intervention.
Keywords: NOX4; NOX5; diabetic kidney disease; fibrosis; inflammation; reactive oxygen species (ROS).
Conflict of interest statement
All authors declare no competing interests.
Figures

Similar articles
-
Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse.Diabetologia. 2019 Sep;62(9):1712-1726. doi: 10.1007/s00125-019-4924-z. Epub 2019 Jun 20. Diabetologia. 2019. PMID: 31222503
-
Independent of Renox, NOX5 Promotes Renal Inflammation and Fibrosis in Diabetes by Activating ROS-Sensitive Pathways.Diabetes. 2022 Jun 1;71(6):1282-1298. doi: 10.2337/db21-1079. Diabetes. 2022. PMID: 35275988
-
Nox (NADPH Oxidase) 1, Nox4, and Nox5 Promote Vascular Permeability and Neovascularization in Retinopathy.Hypertension. 2020 Apr;75(4):1091-1101. doi: 10.1161/HYPERTENSIONAHA.119.14100. Epub 2020 Mar 2. Hypertension. 2020. PMID: 32114846
-
The emerging role of NADPH oxidase NOX5 in vascular disease.Clin Sci (Lond). 2017 May 1;131(10):981-990. doi: 10.1042/CS20160846. Clin Sci (Lond). 2017. PMID: 28473473 Review.
-
Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5.Clin Sci (Lond). 2011 Feb;120(4):131-41. doi: 10.1042/CS20100384. Clin Sci (Lond). 2011. PMID: 21039341 Review.
Cited by
-
Free prostate-specific antigen, total prostate-specific antigen, and their ratio associated with diabetic kidney disease: new evidence from male patients with diabetes in the United States.Eur J Med Res. 2025 Jul 30;30(1):686. doi: 10.1186/s40001-025-02897-6. Eur J Med Res. 2025. PMID: 40739245 Free PMC article.
-
Association between systemic inflammatory indicators on admission and mortality in critically ill patients with diabetic kidney disease based on the MIMIC-IV database: a cohort study.Front Endocrinol (Lausanne). 2025 May 30;16:1503667. doi: 10.3389/fendo.2025.1503667. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40519516 Free PMC article.
References
-
- Sukkar L., Kang A., Hockham C., Young T., Jun M., Foote C., Pecoits-Filho R., Neuen B., Rogers K., Pollock C., et al. Incidence and Associations of Chronic Kidney Disease in Community Participants with Diabetes: A 5-Year Prospective Analysis of the EXTEND45 Study. Diabetes Care. 2020;43:982–990. doi: 10.2337/dc19-1803. - DOI - PMC - PubMed
-
- Zhang H., Rogers K., Sukkar L., Jun M., Kang A., Young T., Campain A., Cass A., Chow C.K., Comino E., et al. Prevalence, incidence and risk factors of diabetes in Australian adults aged >/=45 years: A cohort study using linked routinely-collected data. J. Clin. Transl. Endocrinol. 2020;22:100240. doi: 10.1016/j.jcte.2020.100240. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

