Obesogenic Diet in Mice Leads to Inflammation and Oxidative Stress in the Mother in Association with Sex-Specific Changes in Fetal Development, Inflammatory Markers and Placental Transcriptome
- PMID: 38671859
- PMCID: PMC11047652
- DOI: 10.3390/antiox13040411
Obesogenic Diet in Mice Leads to Inflammation and Oxidative Stress in the Mother in Association with Sex-Specific Changes in Fetal Development, Inflammatory Markers and Placental Transcriptome
Abstract
Background: Obesity during pregnancy is related to adverse maternal and neonatal outcomes. Factors involved in these outcomes may include increased maternal insulin resistance, inflammation, oxidative stress, and nutrient mishandling. The placenta is the primary determinant of fetal outcomes, and its function can be impacted by maternal obesity. The aim of this study on mice was to determine the effect of obesity on maternal lipid handling, inflammatory and redox state, and placental oxidative stress, inflammatory signaling, and gene expression relative to female and male fetal growth.
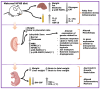
Methods: Female mice were fed control or obesogenic high-fat/high-sugar diet (HFHS) from 9 weeks prior to, and during, pregnancy. On day 18.5 of pregnancy, maternal plasma, and liver, placenta, and fetal serum were collected to examine the immune and redox states. The placental labyrinth zone (Lz) was dissected for RNA-sequencing analysis of gene expression changes.
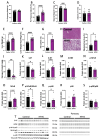
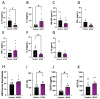
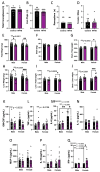
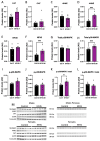
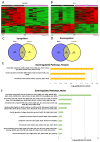
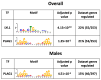
Results: the HFHS diet induced, in the dams, hepatic steatosis, oxidative stress (reduced catalase, elevated protein oxidation) and the activation of pro-inflammatory pathways (p38-MAPK), along with imbalanced circulating cytokine concentrations (increased IL-6 and decreased IL-5 and IL-17A). HFHS fetuses were asymmetrically growth-restricted, showing sex-specific changes in circulating cytokines (GM-CSF, TNF-α, IL-6 and IFN-γ). The morphology of the placenta Lz was modified by an HFHS diet, in association with sex-specific alterations in the expression of genes and proteins implicated in oxidative stress, inflammation, and stress signaling. Placental gene expression changes were comparable to that seen in models of intrauterine inflammation and were related to a transcriptional network involving transcription factors, LYL1 and PLAG1.
Conclusion: This study shows that fetal growth restriction with maternal obesity is related to elevated oxidative stress, inflammatory pathways, and sex-specific placental changes. Our data are important, given the marked consequences and the rising rates of obesity worldwide.
Keywords: inflammation; obesogenic diet; oxidative stress; placenta; pregnancy; sex.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

