Nrf2 Plays a Key Role in Erythropoiesis during Aging
- PMID: 38671902
- PMCID: PMC11047311
- DOI: 10.3390/antiox13040454
Nrf2 Plays a Key Role in Erythropoiesis during Aging
Abstract
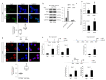
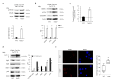
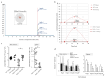
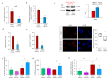
Aging is characterized by increased oxidation and reduced efficiency of cytoprotective mechanisms. Nuclear factor erythroid-2-related factor (Nrf2) is a key transcription factor, controlling the expression of multiple antioxidant proteins. Here, we show that Nrf2-/- mice displayed an age-dependent anemia, due to the combined contributions of reduced red cell lifespan and ineffective erythropoiesis, suggesting a role of Nrf2 in erythroid biology during aging. Mechanistically, we found that the expression of antioxidants during aging is mediated by activation of Nrf2 function by peroxiredoxin-2. The absence of Nrf2 resulted in persistent oxidation and overactivation of adaptive systems such as the unfolded protein response (UPR) system and autophagy in Nrf2-/- mouse erythroblasts. As Nrf2 is involved in the expression of autophagy-related proteins such as autophagy-related protein (Atg) 4-5 and p62, we found impairment of late phase of autophagy in Nrf2-/- mouse erythroblasts. The overactivation of the UPR system and impaired autophagy drove apoptosis of Nrf2-/- mouse erythroblasts via caspase-3 activation. As a proof of concept for the role of oxidation, we treated Nrf2-/- mice with astaxanthin, an antioxidant, in the form of poly (lactic-co-glycolic acid) (PLGA)-loaded nanoparticles (ATS-NPs) to improve its bioavailability. ATS-NPs ameliorated the age-dependent anemia and decreased ineffective erythropoiesis in Nrf2-/- mice. In summary, we propose that Nrf2 plays a key role in limiting age-related oxidation, ensuring erythroid maturation and growth during aging.
Keywords: ATF6; GADD34; PLGA; UPR system; astaxanthin; autophagy; ineffective erythropoiesis; nanoparticles; oxidation; red cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

