Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease
- PMID: 38671903
- PMCID: PMC11047699
- DOI: 10.3390/antiox13040455
Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease
Abstract
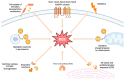
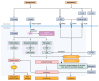
Diabetic kidney disease (DKD) is the principal culprit behind chronic kidney disease (CKD), ultimately developing end-stage renal disease (ESRD) and necessitating costly dialysis or kidney transplantation. The limited therapeutic efficiency among individuals with DKD is a result of our finite understanding of its pathogenesis. DKD is the result of complex interactions between various factors. Oxidative stress is a fundamental factor that can establish a link between hyperglycemia and the vascular complications frequently encountered in diabetes, particularly DKD. It is crucial to recognize the essential and integral role of oxidative stress in the development of diabetic vascular complications, particularly DKD. Hyperglycemia is the primary culprit that can trigger an upsurge in the production of reactive oxygen species (ROS), ultimately sparking oxidative stress. The main endogenous sources of ROS include mitochondrial ROS production, NADPH oxidases (Nox), uncoupled endothelial nitric oxide synthase (eNOS), xanthine oxidase (XO), cytochrome P450 (CYP450), and lipoxygenase. Under persistent high glucose levels, immune cells, the complement system, advanced glycation end products (AGEs), protein kinase C (PKC), polyol pathway, and the hexosamine pathway are activated. Consequently, the oxidant-antioxidant balance within the body is disrupted, which triggers a series of reactions in various downstream pathways, including phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), transforming growth factor beta/p38-mitogen-activated protein kinase (TGF-β/p38-MAPK), nuclear factor kappa B (NF-κB), adenosine monophosphate-activated protein kinase (AMPK), and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling. The disease might persist even if strict glucose control is achieved, which can be attributed to epigenetic modifications. The treatment of DKD remains an unresolved issue. Therefore, reducing ROS is an intriguing therapeutic target. The clinical trials have shown that bardoxolone methyl, a nuclear factor erythroid 2-related factor 2 (Nrf2) activator, blood glucose-lowering drugs, such as sodium-glucose cotransporter 2 inhibitors, and glucagon-like peptide-1 receptor agonists can effectively slow down the progression of DKD by reducing oxidative stress. Other antioxidants, including vitamins, lipoic acid, Nox inhibitors, epigenetic regulators, and complement inhibitors, present a promising therapeutic option for the treatment of DKD. In this review, we conduct a thorough assessment of both preclinical studies and current findings from clinical studies that focus on targeted interventions aimed at manipulating these pathways. We aim to provide a comprehensive overview of the current state of research in this area and identify key areas for future exploration.
Keywords: antioxidant therapy; diabetic kidney disease; molecular pathways; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
Publication types
Grants and funding
- 82370728/National Natural Science Foundation of China
- 81974096/National Natural Science Foundation of China
- 81974097/National Natural Science Foundation of China
- 82170773/National Natural Science Foundation of China
- 82100729/National Natural Science Foundation of China
- 82200808/National Natural Science Foundation of China
- 82200841/National Natural Science Foundation of China
- 82300843/National Natural Science Foundation of China
- 82300786/National Natural Science Foundation of China
- 2021YFC2500200/National Key Research and Development Program of China
- 2023BCB034/Key Research and Development Program of Hubei Province
LinkOut - more resources
Full Text Sources

