Potential New Therapies "ROS-Based" in CLL: An Innovative Paradigm in the Induction of Tumor Cell Apoptosis
- PMID: 38671922
- PMCID: PMC11047475
- DOI: 10.3390/antiox13040475
Potential New Therapies "ROS-Based" in CLL: An Innovative Paradigm in the Induction of Tumor Cell Apoptosis
Abstract
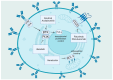
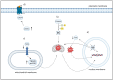
Chronic lymphocytic leukemia, in spite of recent advancements, is still an incurable disease; the majority of patients eventually acquire resistance to treatment through relapses. In all subtypes of chronic lymphocytic leukemia, the disruption of normal B-cell homeostasis is thought to be mostly caused by the absence of apoptosis. Consequently, apoptosis induction is crucial to the management of this illness. Damaged biological components can accumulate as a result of the oxidation of intracellular lipids, proteins, and DNA by reactive oxygen species. It is possible that cancer cells are more susceptible to apoptosis because of their increased production of reactive oxygen species. An excess of reactive oxygen species can lead to oxidative stress, which can harm biological elements like DNA and trigger apoptotic pathways that cause planned cell death. In order to upset the balance of oxidative stress in cells, recent therapeutic treatments in chronic lymphocytic leukemia have focused on either producing reactive oxygen species or inhibiting it. Examples include targets created in the field of nanomedicine, natural extracts and nutraceuticals, tailored therapy using biomarkers, and metabolic targets. Current developments in the complex connection between apoptosis, particularly ferroptosis and its involvement in epigenomics and alterations, have created a new paradigm.
Keywords: apoptosis; chronic lymphocytic leukemia; lymphoproliferative diseases; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Recent advances and future directions in etiopathogenesis and mechanisms of reactive oxygen species in cancer treatment.Pathol Oncol Res. 2023 Oct 18;29:1611415. doi: 10.3389/pore.2023.1611415. eCollection 2023. Pathol Oncol Res. 2023. PMID: 37920248 Free PMC article. Review.
-
Organometallic nucleosides induce non-classical leukemic cell death that is mitochondrial-ROS dependent and facilitated by TCL1-oncogene burden.Mol Cancer. 2015 Jun 4;14:114. doi: 10.1186/s12943-015-0378-1. Mol Cancer. 2015. PMID: 26041471 Free PMC article.
-
Gold Cluster Capped with a BCL-2 Antagonistic Peptide Exerts Synergistic Antitumor Activity in Chronic Lymphocytic Leukemia Cells.ACS Appl Mater Interfaces. 2021 May 12;13(18):21108-21118. doi: 10.1021/acsami.1c05550. Epub 2021 May 4. ACS Appl Mater Interfaces. 2021. PMID: 33942607
-
VDAC1-based peptides: novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia.Cell Death Dis. 2013 Sep 19;4(9):e809. doi: 10.1038/cddis.2013.316. Cell Death Dis. 2013. PMID: 24052077 Free PMC article.
-
Oxidative stress in chronic lymphocytic leukemia: still a matter of debate.Leuk Lymphoma. 2019 Apr;60(4):867-875. doi: 10.1080/10428194.2018.1509317. Epub 2018 Sep 20. Leuk Lymphoma. 2019. PMID: 30234409 Review.
Cited by
-
A pharmacoinformatic approach for studying Atractylodes Lancea DC's anticancer potential and control ROS-mediated apoptosis against prostate cancer cells.Front Oncol. 2025 Feb 5;15:1471110. doi: 10.3389/fonc.2025.1471110. eCollection 2025. Front Oncol. 2025. PMID: 39980567 Free PMC article.
-
The Antitumor Potential of Sicilian Grape Pomace Extract: A Balance between ROS-Mediated Autophagy and Apoptosis.Biomolecules. 2024 Sep 3;14(9):1111. doi: 10.3390/biom14091111. Biomolecules. 2024. PMID: 39334877 Free PMC article.
-
The Novel Anti-Cancer Agent, SpiD3, Is Cytotoxic in CLL Cells Resistant to Ibrutinib or Venetoclax.Hemato. 2024 Sep;5(3):321-339. doi: 10.3390/hemato5030024. Epub 2024 Aug 27. Hemato. 2024. PMID: 39450301 Free PMC article.
-
Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma.Cancers (Basel). 2025 Jul 14;17(14):2331. doi: 10.3390/cancers17142331. Cancers (Basel). 2025. PMID: 40723215 Free PMC article. Review.
-
Cellular and molecular mechanisms of arenobufagin in cancer therapy: a systematic review.Discov Oncol. 2025 Jul 1;16(1):1207. doi: 10.1007/s12672-025-03052-7. Discov Oncol. 2025. PMID: 40591087 Free PMC article. Review.
References
-
- Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Döhner H., Hillmen P., Keating M., Montserrat E., Chiorazzi N., et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. - DOI - PubMed
-
- Swerdlow S.H., Campo E., Pileri S.A., Swerdlow S.H., Campo E., Pileri S.A., Lee Harris N., Stein H., Siebert R., Advani T., et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. - DOI - PMC - PubMed
-
- Eichhorst B., Robak T., Montserrat E., Ghia P., Niemann C.U., Kater A.P., Gregor M., Cymbalista F., Buske C., Hillmen P., et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:23–33. doi: 10.1016/j.annonc.2020.09.019. - DOI - PubMed
-
- Rawstron A.C., Kreuzer K.A., Soosapilla A., Spacek M., Stehlikova O., Gambell P., McIver-Brown N., Villamor N., Psarra K., Arroz M., et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytom. B Clin. Cytom. 2018;94:121–128. doi: 10.1002/cyto.b.21595. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

