Uncovering the Cardioprotective Potential of Diacerein in Doxorubicin Cardiotoxicity: Mitigating Ferritinophagy-Mediated Ferroptosis via Upregulating NRF2/SLC7A11/GPX4 Axis
- PMID: 38671940
- PMCID: PMC11047461
- DOI: 10.3390/antiox13040493
Uncovering the Cardioprotective Potential of Diacerein in Doxorubicin Cardiotoxicity: Mitigating Ferritinophagy-Mediated Ferroptosis via Upregulating NRF2/SLC7A11/GPX4 Axis
Abstract
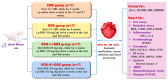
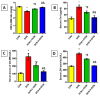
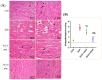
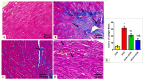
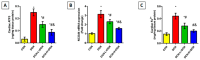
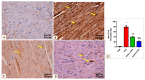
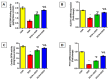
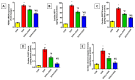
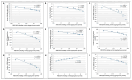
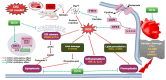
Doxorubicin (DOX)-induced cardiotoxicity (DIC) is a life-threatening clinical issue with limited preventive approaches, posing a substantial challenge to cancer survivors. The anthraquinone diacerein (DCN) exhibits significant anti-inflammatory, anti-proliferative, and antioxidant actions. Its beneficial effects on DIC have yet to be clarified. Therefore, this study investigated DCN's cardioprotective potency and its conceivable molecular targets against DIC. Twenty-eight Wister rats were assigned to CON, DOX, DCN-L/DOX, and DCN-H/DOX groups. Serum cardiac damage indices, iron assay, oxidative stress, inflammation, endoplasmic reticulum (ER) stress, apoptosis, ferritinophagy, and ferroptosis-related biomarkers were estimated. Nuclear factor E2-related factor 2 (NRF2) DNA-binding activity and phospho-p53 immunoreactivity were assessed. DCN administration effectively ameliorated DOX-induced cardiac cytomorphological abnormalities. Additionally, DCN profoundly combated the DOX-induced labile iron pool expansion alongside its consequent lethal lipid peroxide overproduction, whereas it counteracted ferritinophagy and enhanced iron storage. Indeed, DCN valuably reinforced the cardiomyocytes' resistance to ferroptosis, mainly by restoring the NRF2/solute carrier family 7 member 11 (SLC7A11)/glutathione peroxidase 4 (GPX4) signaling axis. Furthermore, DCN abrogated the cardiac oxidative damage, inflammatory response, ER stress, and cardiomyocyte apoptosis elicited by DOX. In conclusion, for the first time, our findings validated DCN's cardioprotective potency against DIC based on its antioxidant, anti-inflammatory, anti-ferroptotic, and anti-apoptotic imprint, chiefly mediated by the NRF2/SLC7A11/GPX4 axis. Accordingly, DCN could represent a promising therapeutic avenue for patients under DOX-dependent chemotherapy.
Keywords: diacerein; doxorubicin cardiotoxicity; ferritin heavy chain polypeptide 1; ferroptosis; iron; nuclear factor E2-related factor 2/solute carrier family 7 member 11/ glutathione peroxidase 4 axis; nuclear receptor coactivator 4-dependent ferritinophagy; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Wu C., Du M., Yu R., Cheng Y., Wu B., Fu J., Tan W., Zhou Q., Balawi E., Liao Z. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free. Radic. Biol. Med. 2022;178:271–294. doi: 10.1016/j.freeradbiomed.2021.12.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

