How Lifetime Evolution of Parkinson's Disease Could Shape Clinical Trial Design: A Shared Patient-Clinician Viewpoint
- PMID: 38672010
- PMCID: PMC11048137
- DOI: 10.3390/brainsci14040358
How Lifetime Evolution of Parkinson's Disease Could Shape Clinical Trial Design: A Shared Patient-Clinician Viewpoint
Abstract
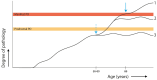
Parkinson's disease (PD) has a long, heterogeneous, pre-diagnostic phase, during which pathology insidiously accumulates. Increasing evidence suggests that environmental and lifestyle factors in early life contribute to disease risk and progression. Thanks to the extensive study of this pre-diagnostic phase, the first prevention trials of PD are being designed. However, the highly heterogenous evolution of the disease across the life course is not yet sufficiently taken into account. This could hamper clinical trial success in the advent of biological disease definitions. In an interdisciplinary patient-clinician study group, we discussed how an approach that incorporates the lifetime evolution of PD may benefit the design of disease-modifying trials by impacting population, target and outcome selection. We argue that the timepoint of exposure to risk and protective factors plays a critical role in PD subtypes, influencing population selection. In addition, recent developments in differential disease mechanisms, aided by biological disease definitions, could impact optimal treatment targets. Finally, multimodal biomarker panels using this lifetime approach will likely be most sensitive as progression markers for more personalized trials. We believe that the lifetime evolution of PD should be considered in the design of clinical trials, and that such initiatives could benefit from more patient-clinician partnerships.
Keywords: Parkinson’s disease; disease-modifying treatment; lifetime research; patient–clinician collaboration.
Conflict of interest statement
Dr. Sirwan Darweesh currently serves on the editorial board of
Figures

Similar articles
-
Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation.Hum Gene Ther. 2001 Aug 10;12(12):1589-91. Hum Gene Ther. 2001. PMID: 11529246 Clinical Trial.
-
How should we be using biomarkers in trials of disease modification in Parkinson's disease?Brain. 2023 Dec 1;146(12):4845-4869. doi: 10.1093/brain/awad265. Brain. 2023. PMID: 37536279 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
What is the Clinical Significance of Cerebrospinal Fluid Biomarkers in Parkinson's disease? Is the Significance Diagnostic or Prognostic?Exp Neurobiol. 2014 Dec;23(4):352-64. doi: 10.5607/en.2014.23.4.352. Epub 2014 Dec 12. Exp Neurobiol. 2014. PMID: 25548535 Free PMC article. Review.
-
GM1 Ganglioside as a Disease-Modifying Therapeutic for Parkinson's Disease: A Multi-Functional Glycosphingolipid That Targets Multiple Parkinson's Disease-Relevant Pathogenic Mechanisms.Int J Mol Sci. 2023 May 24;24(11):9183. doi: 10.3390/ijms24119183. Int J Mol Sci. 2023. PMID: 37298133 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

