Glucose Promotes EMMPRIN/CD147 and the Secretion of Pro-Angiogenic Factors in a Co-Culture System of Endothelial Cells and Monocytes
- PMID: 38672062
- PMCID: PMC11047830
- DOI: 10.3390/biomedicines12040706
Glucose Promotes EMMPRIN/CD147 and the Secretion of Pro-Angiogenic Factors in a Co-Culture System of Endothelial Cells and Monocytes
Abstract
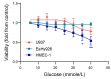
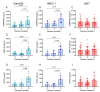
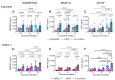
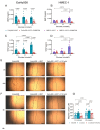
Vascular complications in Type 2 diabetes mellitus (T2DM) patients increase morbidity and mortality. In T2DM, angiogenesis is impaired and can be enhanced or reduced in different tissues ("angiogenic paradox"). The present study aimed to delineate differences between macrovascular and microvascular endothelial cells that might explain this paradox. In a monoculture system of human macrovascular (EaHy926) or microvascular (HMEC-1) endothelial cell lines and a monocytic cell line (U937), high glucose concentrations (25 mmole/L) increased the secretion of the pro-angiogenic factors CD147/EMMPRIN, VEGF, and MMP-9 from both endothelial cells, but not from monocytes. Co-cultures of EaHy926/HMEC-1 with U937 enhanced EMMPRIN and MMP-9 secretion, even in low glucose concentrations (5.5 mmole/L), while in high glucose HMEC-1 co-cultures enhanced all three factors. EMMPRIN mediated these effects, as the addition of anti-EMMPRIN antibody decreased VEGF and MMP-9 secretion, and inhibited the angiogenic potential assessed through the wound assay. Thus, the minor differences between the macrovascular and microvascular endothelial cells cannot explain the angiogenic paradox. Metformin, a widely used drug for the treatment of T2DM, inhibited EMMPRIN, VEGF, and MMP-9 secretion in high glucose concentration, and the AMPK inhibitor dorsomorphin enhanced it. Thus, AMPK regulates EMMPRIN, a key factor in diabetic angiogenesis, suggesting that targeting EMMPRIN may help in the treatment of diabetic vascular complications.
Keywords: AMPK; CD147/EMMPRIN; diabetes mellitus; dorsomorphin/compound C; endothelial cells; glucose; metformin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Tocilizumab (TCZ) Decreases Angiogenesis in Rheumatoid Arthritis Through Its Regulatory Effect on miR-146a-5p and EMMPRIN/CD147.Front Immunol. 2021 Dec 15;12:739592. doi: 10.3389/fimmu.2021.739592. eCollection 2021. Front Immunol. 2021. PMID: 34975837 Free PMC article.
-
Tumor cell-macrophage interactions increase angiogenesis through secretion of EMMPRIN.Front Physiol. 2013 Jul 12;4:178. doi: 10.3389/fphys.2013.00178. eCollection 2013. Front Physiol. 2013. PMID: 23874303 Free PMC article.
-
Soluble CD147 regulates endostatin via its effects on the activities of MMP-9 and secreted proteasome 20S.Front Immunol. 2024 Jan 22;15:1319939. doi: 10.3389/fimmu.2024.1319939. eCollection 2024. Front Immunol. 2024. PMID: 38318187 Free PMC article.
-
EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair.Biochimie. 2005 Mar-Apr;87(3-4):361-8. doi: 10.1016/j.biochi.2004.09.023. Biochimie. 2005. PMID: 15781323 Review.
-
Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression.Thromb Haemost. 2005 Feb;93(2):199-204. doi: 10.1160/TH04-08-0536. Thromb Haemost. 2005. PMID: 15711733 Review.
Cited by
-
Hydrogen Sulfide Modulation of Matrix Metalloproteinases and CD147/EMMPRIN: Mechanistic Pathways and Impact on Atherosclerosis Progression.Biomedicines. 2024 Aug 26;12(9):1951. doi: 10.3390/biomedicines12091951. Biomedicines. 2024. PMID: 39335465 Free PMC article. Review.
-
Metabolic Function and Therapeutic Potential of CD147 for Hematological Malignancies: An Overview.Int J Mol Sci. 2024 Aug 23;25(17):9178. doi: 10.3390/ijms25179178. Int J Mol Sci. 2024. PMID: 39273126 Free PMC article. Review.
-
Expression of MMP-14 and CD147 in Gingival Tissue of Patients With and Without Diabetes Mellitus Type II.Diagnostics (Basel). 2025 Mar 3;15(5):609. doi: 10.3390/diagnostics15050609. Diagnostics (Basel). 2025. PMID: 40075856 Free PMC article.
-
Cyclophilin A and C are the Main Components of Extracellular Vesicles in Response to Hyperglycemia in BV2 Microglial Cells.Mol Neurobiol. 2025 Aug;62(8):10349-10366. doi: 10.1007/s12035-025-04921-6. Epub 2025 Apr 8. Mol Neurobiol. 2025. PMID: 40199808 Free PMC article.
References
-
- Ong K.L., Stafford L.K., McLaughlin S.A., Boyko E.J., Vollset S.E., Smith A.E., Dalton B.E., Duprey J., Cruz J.A., Hagins H., et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203–234. doi: 10.1016/S0140-6736(23)01301-6. - DOI - PMC - PubMed
-
- Giri B., Dey S., Das T., Sarkar M., Banerjee J., Dash S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018;107:306–328. doi: 10.1016/j.biopha.2018.07.157. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

