Molecular Pathology of Thyroid Tumors: Essential Points to Comprehend Regarding the Latest WHO Classification
- PMID: 38672067
- PMCID: PMC11048493
- DOI: 10.3390/biomedicines12040712
Molecular Pathology of Thyroid Tumors: Essential Points to Comprehend Regarding the Latest WHO Classification
Abstract
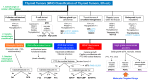
In 2022, the new WHO Classification of Endocrine and Neuroendocrine Tumors, Fifth Edition (beta version) (WHO 5th), was published. Large-scale genomic analyses such as The Cancer Genome Atlas (TCGA) have revealed the importance of understanding the molecular genetics of thyroid tumors. Consequently, the WHO 5th was fundamentally revised, resulting in a systematic classification based on the cell of origin of tumors and their clinical risk. This paper outlines the following critical points of the WHO 5th. 1. Genetic mutations in follicular cell-derived neoplasms (FDNs) highlight the role of mutations in the MAP kinase pathway, including RET, RAS, and BRAF, as drivers of carcinogenesis. Differentiated thyroid cancers such as follicular thyroid carcinoma (FTC) and papillary thyroid carcinoma (PTC) have specific genetic alterations that correlate with morphological classifications: RAS-like tumors (RLTs) and BRAF p.V600E-like tumors (BLTs), respectively. 2. The framework for benign lesions has been revised. The WHO 5th introduces a new category: "developmental abnormalities". Benign FDNs comprise "thyroid follicular nodular disease", follicular thyroid adenoma (FTA), FTA with papillary architecture, and oncocytic adenoma (OA). "Hürthle cell adenoma/carcinoma" is renamed oncocytic adenoma/carcinoma of the thyroid (OA/OCA), which can be distinguished from FTA/FTC by its unique genetic background. 3. Low-risk tumors include NIFTP, TT-UMP, and HTT, and they have an extremely low malignant potential or an uncertain malignant potential. 4. PTC histological variants are reclassified as "subtypes" in the WHO 5th. 5. The concept of high-grade carcinomas is introduced, encompassing poorly differentiated thyroid carcinoma (PDTC), differentiated high-grade thyroid carcinoma (DHGTC), and high-grade medullary thyroid carcinoma (MTC). 6. Squamous cell carcinoma is included in anaplastic thyroid carcinoma (ATC) in the WHO 5th due to their shared genetic and prognostic features. 7. Other miscellaneous tumors are categorized as salivary-gland-type carcinomas of the thyroid, thyroid tumors of uncertain histogenesis, thymic tumors within the thyroid, and embryonal thyroid neoplasms. The WHO 5th thus emphasizes the importance of classifying tumors based on both genetic abnormalities and histomorphology. This approach aids in achieving accurate pathological diagnosis and facilitates the early selection of appropriate treatment options, including molecular targeted therapies.
Keywords: WHO classification; genomic alterations; pathological diagnosis; thyroid cancer.
Conflict of interest statement
The author declares no conflicts of interest.
Figures



References
-
- Kamma H., Kameyama K., Kondo T., Imamura Y., Nakashima M., Chiba T., Hirokawa M. Pathological diagnosis of general rules for the description of thyroid cancer by Japanese Society of Thyroid Pathology and Japan Association of Endocrine Surgery. Endocr. J. 2022;69:139–154. doi: 10.1507/endocrj.EJ21-0388. - DOI - PubMed
-
- Lloyd R.V., Osamura R.Y., Klöppel G., Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th ed. IARC; Lyon, France: 2017.
-
- Asa S.L., Baloch Z.W., de Krijger R.R., Erickson L.A., Ghossein R.A., Hyrcza M.D., Klimstra D.S., Mete O., Nosé V., Osamura R.Y., et al. WHO Classification of Tumours of Endocrine Organs [Internet] 5th ed. IARC; Lyon, France: 2022.
-
- Nikiforov Y.E., Biddinger P.W., Thompson L.D.R. Diagnostic Pathology and Molecular Genetics of the Thyroid. 3rd ed. Wolters Kluwer; Alphen aan den Rijn, The Netherlands: 2020. pp. 347–387.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

