NMDA Receptor Antagonist Memantine Ameliorates Experimental Autoimmune Encephalomyelitis in Aged Rats
- PMID: 38672073
- PMCID: PMC11047843
- DOI: 10.3390/biomedicines12040717
NMDA Receptor Antagonist Memantine Ameliorates Experimental Autoimmune Encephalomyelitis in Aged Rats
Abstract
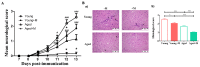
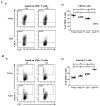
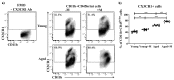
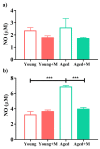
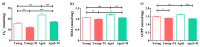
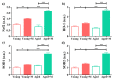
Aging is closely related to the main aspects of multiple sclerosis (MS). The average age of the MS population is increasing and the number of elderly MS patients is expected to increase. In addition to neurons, N-methyl-D-aspartate receptors (NMDARs) are also expressed on non-neuronal cells, such as immune cells. The aim of this study was to investigate the role of NMDARs in experimental autoimmune encephalomyelitis (EAE) in young and aged rats. Memantine, a non-competitive NMDAR antagonist, was administered to young and aged Dark Agouti rats from day 7 after immunization. Antagonizing NMDARs had a more favourable effect on clinical disease, reactivation, and apoptosis of CD4+ T cells in the target organ of aged EAE rats. The expression of the fractalkine receptor CX3CR1 was increased in memantine-treated rats, but to a greater extent in aged rats. Additionally, memantine increased Nrf2 and Nrf2-regulated enzymes' mRNA expression in brain tissue. The concentrations of superoxide anion radicals, malondialdehyde, and advanced oxidation protein products in brain tissue were consistent with previous results. Overall, our results suggest that NMDARs play a more important role in the pathogenesis of EAE in aged than in young rats.
Keywords: EAE; NMDARs; aging; memantine; multiple sclerosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Memantine Modulates Oxidative Stress in the Rat Brain following Experimental Autoimmune Encephalomyelitis.Int J Mol Sci. 2021 Oct 20;22(21):11330. doi: 10.3390/ijms222111330. Int J Mol Sci. 2021. PMID: 34768760 Free PMC article.
-
Inhibition of NR2B-Containing N-methyl-D-Aspartate Receptors (NMDARs) in Experimental Autoimmune Encephalomyelitis, a Model of Multiple Sclerosis.Iran J Pharm Res. 2014 Spring;13(2):695-705. Iran J Pharm Res. 2014. PMID: 25237366 Free PMC article.
-
Age-associated changes in rat immune system: lessons learned from experimental autoimmune encephalomyelitis.Exp Gerontol. 2014 Oct;58:179-97. doi: 10.1016/j.exger.2014.08.005. Epub 2014 Aug 13. Exp Gerontol. 2014. PMID: 25128713
-
The chemical biology of clinically tolerated NMDA receptor antagonists.J Neurochem. 2006 Jun;97(6):1611-26. doi: 10.1111/j.1471-4159.2006.03991.x. J Neurochem. 2006. PMID: 16805772 Review.
-
Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: implications for preventive treatments of ischemic stroke and late-onset Alzheimer's disease.Mol Neurodegener. 2023 Jul 3;18(1):43. doi: 10.1186/s13024-023-00636-1. Mol Neurodegener. 2023. PMID: 37400870 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

