Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway
- PMID: 38672086
- PMCID: PMC11048106
- DOI: 10.3390/biomedicines12040730
Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway
Abstract
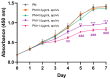
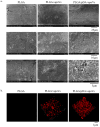
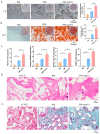
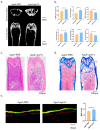
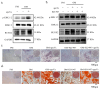
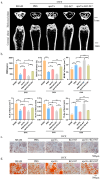
Osteoporosis is a common degenerative bone disease. The treatment of osteoporosis remains a clinical challenge in light of the increasing aging population. Human dental pulp stem cells (DPSCs), a type of mesenchymal stem cells (MSCs), are easy to obtain and have a high proliferation ability, playing an important role in the treatment of osteoporosis. However, MSCs undergo apoptosis within a short time when used in vivo; therefore, apoptotic vesicles (apoVs) have attracted increasing attention. Currently, the osteogenic effect of DPSC-derived apoVs is unknown; therefore, this study aimed to determine the role of DPSC-derived apoVs and their potential mechanisms in bone regeneration. We found that MSCs could take up DPSC-derived apoVs, which then promoted MSC osteogenesis in vitro. Moreover, apoVs could increase the trabecular bone count and bone mineral density in the mouse osteoporosis model and could promote bone formation in rat cranial defects in vivo. Mechanistically, apoVs promoted MSC osteogenesis by activating the extracellular regulated kinase (ERK)1/2 signaling pathway. Consequently, we propose a novel therapy comprising DPSC-derived apoVs, representing a promising approach to treat bone loss and bone defects.
Keywords: ERK1/2 signaling pathway; apoptotic vesicles; bone defect; dental pulp stem cells; osteogenic differentiation; osteoporosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










Similar articles
-
Macrophage-derived apoptotic vesicles regulate fate commitment of mesenchymal stem cells via miR155.Stem Cell Res Ther. 2022 Jul 16;13(1):323. doi: 10.1186/s13287-022-03004-w. Stem Cell Res Ther. 2022. PMID: 35842708 Free PMC article.
-
Apoptotic vesicles derived from human red blood cells promote bone regeneration via carbonic anhydrase 1.Cell Prolif. 2024 Feb;57(2):e13547. doi: 10.1111/cpr.13547. Epub 2023 Sep 11. Cell Prolif. 2024. PMID: 37697490 Free PMC article.
-
Platelet-Derived Apoptotic Vesicles Promote Bone Regeneration via Golgi Phosphoprotein 2 (GOLPH2)-AKT Signaling Axis.ACS Nano. 2023 Dec 26;17(24):25070-25090. doi: 10.1021/acsnano.3c07717. Epub 2023 Dec 4. ACS Nano. 2023. PMID: 38047915 Free PMC article.
-
Apoptotic Vesicles of MSCs: The Natural Therapeutic Agents and Bio-Vehicles for Targeting Drug Delivery.Small. 2023 Nov;19(47):e2301671. doi: 10.1002/smll.202301671. Epub 2023 Jul 25. Small. 2023. PMID: 37491784 Review.
-
Molecules Inducing Dental Stem Cells Differentiation and Bone Regeneration: State of the Art.Int J Mol Sci. 2023 Jun 8;24(12):9897. doi: 10.3390/ijms24129897. Int J Mol Sci. 2023. PMID: 37373044 Free PMC article. Review.
Cited by
-
The existing evidence for the use of extracellular vesicles in the treatment of osteoporosis: a review.Int J Surg. 2025 May 1;111(5):3414-3429. doi: 10.1097/JS9.0000000000002339. Int J Surg. 2025. PMID: 40085758 Free PMC article. Review.
-
Unlocking the potential of stem cell-derived extracellular vesicles in osteoporosis therapy: a systematic review and meta-analysis of preclinical studies.J Transl Med. 2025 Jun 18;23(1):683. doi: 10.1186/s12967-025-06654-5. J Transl Med. 2025. PMID: 40533796 Free PMC article. Review.
-
Apoptotic mesenchymal stem cells and their secreted apoptotic extracellular vesicles: therapeutic applications and mechanisms.Stem Cell Res Ther. 2025 Feb 21;16(1):78. doi: 10.1186/s13287-025-04211-x. Stem Cell Res Ther. 2025. PMID: 39985021 Free PMC article. Review.
-
Tailored Extracellular Vesicles from Dental Stem Cells: Advances in Specific Modifications for Enhanced Therapeutic Applications.Int J Nanomedicine. 2025 Jun 26;20:8327-8341. doi: 10.2147/IJN.S528190. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40599394 Free PMC article. Review.
References
-
- Zhao P., Xiao L., Peng J., Peng J., Qian Y.Q., Huang C.C. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3962–3970. - PubMed
-
- Jiang H.T., Ran C.C., Liao Y.P., Zhu J.H., Wang H., Deng R., Nie M., He B.C., Deng Z.L. IGF-1 reverses the osteogenic inhibitory effect of dexamethasone on BMP9-induced osteogenic differentiation in mouse embryonic fibroblasts via PI3K/AKT/COX-2 pathway. J. Steroid Biochem. Mol. Biol. 2019;191:105363. doi: 10.1016/j.jsbmb.2019.04.012. - DOI - PubMed
-
- Wang C., Liu Y., Hu X., Shang X., Ma S., Guo H., Ma X., Cai D., Hu Z., Zhao Y., et al. Titanium dioxide nanotubes increase purinergic receptor P2Y6 expression and activate its downstream PKCα-ERK1/2 pathway in bone marrow mesenchymal stem cells under osteogenic induction. Acta Biomater. 2023;157:670–682. doi: 10.1016/j.actbio.2022.11.045. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

