Detection of ER Stress in iPSC-Derived Neurons Carrying the p.N370S Mutation in the GBA1 Gene
- PMID: 38672099
- PMCID: PMC11047942
- DOI: 10.3390/biomedicines12040744
Detection of ER Stress in iPSC-Derived Neurons Carrying the p.N370S Mutation in the GBA1 Gene
Abstract
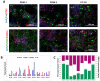
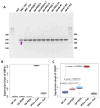
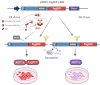
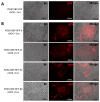
Endoplasmic reticulum (ER) stress is involved in the pathogenesis of many human diseases, such as cancer, type 2 diabetes, kidney disease, atherosclerosis and neurodegenerative diseases, in particular Parkinson's disease (PD). Since there is currently no treatment for PD, a better understanding of the molecular mechanisms underlying its pathogenesis, including the mechanisms of the switch from adaptation in the form of unfolded protein response (UPR) to apoptosis under ER stress conditions, may help in the search for treatment methods. Genetically encoded biosensors based on fluorescent proteins are suitable tools that facilitate the study of living cells and visualization of molecular events in real time. The combination of technologies to generate patient-specific iPSC lines and genetically encoded biosensors allows the creation of cell models with new properties. Using CRISPR-Cas9-mediated homologous recombination at the AAVS1 locus of iPSC with the genetic variant p.N370S (rs76763715) in the GBA1 gene, we created a cell model designed to study the activation conditions of the IRE1-XBP1 cascade of the UPR system. The cell lines obtained have a doxycycline-dependent expression of the genetically encoded biosensor XBP1-TagRFP, possess all the properties of human pluripotent cells, and can be used to test physical conditions and chemical compounds that affect the development of ER stress, the functioning of the UPR system, and in particular, the IRE1-XBP1 cascade.
Keywords: CRISPR/Cas9; ER stress; GBA1; Parkinson’s disease; biosensors; endoplasmic reticulum; induced pluripotent stem cells.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures




Similar articles
-
N370S-GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson's disease.Mov Disord. 2017 Oct;32(10):1409-1422. doi: 10.1002/mds.27119. Epub 2017 Aug 5. Mov Disord. 2017. PMID: 28779532
-
Neuronal activity-dependent local activation of dendritic unfolded protein response promotes expression of brain-derived neurotrophic factor in cell soma.J Neurochem. 2018 Jan;144(1):35-49. doi: 10.1111/jnc.14221. Epub 2017 Oct 16. J Neurochem. 2018. PMID: 28921568 Free PMC article.
-
The balance between adaptive and apoptotic unfolded protein responses regulates β-cell death under ER stress conditions through XBP1, CHOP and JNK.Mol Cell Endocrinol. 2015 Sep 15;413:189-201. doi: 10.1016/j.mce.2015.06.025. Epub 2015 Jun 30. Mol Cell Endocrinol. 2015. PMID: 26135354
-
Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells.Mol Metab. 2017 Jul 12;6(9):1024-1039. doi: 10.1016/j.molmet.2017.06.001. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951826 Free PMC article. Review.
-
Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain.Cell Calcium. 2003 Oct-Nov;34(4-5):365-83. doi: 10.1016/s0143-4160(03)00139-8. Cell Calcium. 2003. PMID: 12909082 Review.
Cited by
-
Innovative Atherosclerosis Models: Advancing Pathophysiology and Translational Research.Research (Wash D C). 2025 Feb 20;8:0617. doi: 10.34133/research.0617. eCollection 2025. Research (Wash D C). 2025. PMID: 40046514 Free PMC article. Review.
-
Transgenic iPSC Lines with Genetically Encoded MitoTimer to Study Mitochondrial Biogenesis in Dopaminergic Neurons with Tauopathy.Biomedicines. 2025 Feb 21;13(3):550. doi: 10.3390/biomedicines13030550. Biomedicines. 2025. PMID: 40149527 Free PMC article.
-
Pluripotent Stem Cells: Recent Advances and Emerging Trends.Biomedicines. 2025 Mar 21;13(4):765. doi: 10.3390/biomedicines13040765. Biomedicines. 2025. PMID: 40299329 Free PMC article.
References
-
- Subach O.M., Kunitsyna T.A., Mineyeva O.A., Lazutkin A.A., Bezryadnov D.V., Barykina N.V., Piatkevich K.D., Ermakova Y.G., Bilan D.S., Belousov V.V., et al. Slowly reducible genetically encoded green fluorescent indicator for in vivo and ex vivo visualization of hydrogen peroxide. Int. J. Mol. Sci. 2019;20:3138. doi: 10.3390/ijms20133138. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

