USP7 Deregulation Impairs S Phase Specific DNA Repair after Irradiation in Breast Cancer Cells
- PMID: 38672118
- PMCID: PMC11047985
- DOI: 10.3390/biomedicines12040762
USP7 Deregulation Impairs S Phase Specific DNA Repair after Irradiation in Breast Cancer Cells
Abstract
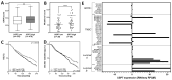
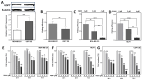
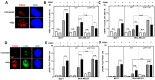
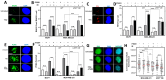
The ubiquitin specific protease 7 (USP7) is a deubiquitinating enzyme with numerous substrates. Aberrant expression of USP7 is associated with tumor progression. This study aims to investigate how a deregulated USP7 expression affects chromosomal instability and prognosis of breast cancer patients in silico and radiosensitivity and DNA repair in breast cancer cells in vitro. The investigations in silico were performed using overall survival and USP7 mRNA expression data of breast cancer patients. The results showed that a high USP7 expression was associated with increased chromosomal instability and decreased overall survival. The in vitro experiments were performed in a luminal and a triple-negative breast cancer cell line. Proliferation, DNA repair, DNA replication stress, and survival after USP7 overexpression or inhibition and irradiation were analyzed. Both, USP7 inhibition and overexpression resulted in decreased cellular survival, distinct radiosensitization and an increased number of residual DNA double-strand breaks in the S phase following irradiation. RAD51 recruitment and base incorporation were decreased after USP7 inhibition plus irradiation and more single-stranded DNA was detected. The results show that deregulation of USP7 activity disrupts DNA repair in the S phase by increasing DNA replication stress and presents USP7 as a promising target to overcome the radioresistance of breast tumors.
Keywords: DNA repair; USP7; breast cancer; genomic instability; radiosensitization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Ubiquitin-specific protease 7 regulates nucleotide excision repair through deubiquitinating XPC protein and preventing XPC protein from undergoing ultraviolet light-induced and VCP/p97 protein-regulated proteolysis.J Biol Chem. 2014 Sep 26;289(39):27278-27289. doi: 10.1074/jbc.M114.589812. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118285 Free PMC article.
-
USP7 Is a Master Regulator of Genome Stability.Front Cell Dev Biol. 2020 Aug 5;8:717. doi: 10.3389/fcell.2020.00717. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850836 Free PMC article. Review.
-
Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation.Proc Natl Acad Sci U S A. 2014 Mar 25;111(12):4536-41. doi: 10.1073/pnas.1402604111. Epub 2014 Mar 10. Proc Natl Acad Sci U S A. 2014. PMID: 24616504 Free PMC article.
-
DUB3 and USP7 de-ubiquitinating enzymes control replication inhibitor Geminin: molecular characterization and associations with breast cancer.Oncogene. 2017 Aug 17;36(33):4802-4809. doi: 10.1038/onc.2017.21. Epub 2017 Mar 13. Oncogene. 2017. PMID: 28288134
-
Ubiquitin-specific protease 7 (USP7): an emerging drug target for cancer treatment.Expert Opin Ther Targets. 2023 Jul-Dec;27(11):1043-1058. doi: 10.1080/14728222.2023.2266571. Epub 2023 Dec 7. Expert Opin Ther Targets. 2023. PMID: 37789645 Review.
Cited by
-
The Role and Mechanism of Deubiquitinase USP7 in Tumor-Associated Inflammation.Biomedicines. 2024 Nov 29;12(12):2734. doi: 10.3390/biomedicines12122734. Biomedicines. 2024. PMID: 39767641 Free PMC article. Review.
References
-
- Ades F., Zardavas D., Bozovic-Spasojevic I., Pugliano L., Fumagalli D., de Azambuja E., Viale G., Sotiriou C., Piccart M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. - DOI - PubMed
-
- O’Brien K.M., Cole S.R., Tse C.-K., Perou C.M., Carey L.A., Foulkes W.D., Dressler L.G., Geradts J., Millikan R.C. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

