Plasma Proteomics Elucidated a Protein Signature in COVID-19 Patients with Comorbidities and Early-Diagnosis Biomarkers
- PMID: 38672194
- PMCID: PMC11048573
- DOI: 10.3390/biomedicines12040840
Plasma Proteomics Elucidated a Protein Signature in COVID-19 Patients with Comorbidities and Early-Diagnosis Biomarkers
Abstract
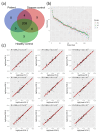
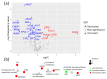
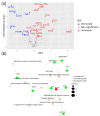
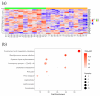
Despite great scientific efforts, deep understanding of coronavirus-19 disease (COVID-19) immunopathology and clinical biomarkers remains a challenge. Pre-existing comorbidities increase the mortality rate and aggravate the exacerbated immune response against the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, which can result in more severe symptoms as well as long-COVID and post-COVID complications. In this study, we applied proteomics analysis of plasma samples from 28 patients with SARS-CoV-2, with and without pre-existing comorbidities, as well as their corresponding controls to determine the systemic protein changes caused by the SARS-CoV-2 infection. As a result, the protein signature shared amongst COVID-19 patients with comorbidities was revealed to be characterized by alterations in the coagulation and complement pathways, acute-phase response proteins, tissue damage and remodeling, as well as cholesterol metabolism. These altered proteins may play a relevant role in COVID-19 pathophysiology. Moreover, several novel potential biomarkers for early diagnosis of the SARS-CoV-2 infection were detected, such as increased levels of keratin K22E, extracellular matrix protein-1 (ECM1), and acute-phase response protein α-2-antiplasmin (A2AP). Importantly, elevated A2AP may contribute to persistent clotting complications associated with the long-COVID syndrome in patients with comorbidities. This study provides new insights into COVID-19 pathogenesis and proposes novel potential biomarkers for early diagnosis that could be facilitated for clinical application by further validation studies.
Keywords: COVID-19; LC-MS/MS; SARS-CoV-2; acute-phase reaction; biomarker; comorbidity; complement; diagnosis; inflammation; plasma proteomics.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Plasma Proteomics Unveil Novel Immune Signatures and Biomarkers upon SARS-CoV-2 Infection.Int J Mol Sci. 2023 Mar 27;24(7):6276. doi: 10.3390/ijms24076276. Int J Mol Sci. 2023. PMID: 37047248 Free PMC article.
-
Targeted proteomics identifies circulating biomarkers associated with active COVID-19 and post-COVID-19.Front Immunol. 2022 Nov 3;13:1027122. doi: 10.3389/fimmu.2022.1027122. eCollection 2022. Front Immunol. 2022. PMID: 36405747 Free PMC article.
-
Unraveling the molecular crosstalk and immune landscape between COVID-19 infections and ischemic heart failure comorbidity: New insights into diagnostic biomarkers and therapeutic approaches.Cell Signal. 2023 Dec;112:110909. doi: 10.1016/j.cellsig.2023.110909. Epub 2023 Sep 28. Cell Signal. 2023. PMID: 37777104
-
Pathogenesis-directed therapy of 2019 novel coronavirus disease.J Med Virol. 2021 Mar;93(3):1320-1342. doi: 10.1002/jmv.26610. Epub 2020 Nov 10. J Med Virol. 2021. PMID: 33073355 Review.
-
Comorbidities and inflammation associated with ovarian cancer and its influence on SARS-CoV-2 infection.J Ovarian Res. 2021 Feb 25;14(1):39. doi: 10.1186/s13048-021-00787-z. J Ovarian Res. 2021. PMID: 33632295 Free PMC article. Review.
Cited by
-
Utility of Protein Markers in COVID-19 Patients.Int J Mol Sci. 2025 Jan 14;26(2):653. doi: 10.3390/ijms26020653. Int J Mol Sci. 2025. PMID: 39859366 Free PMC article. Review.
-
The New Occurrence of Antiphospholipid Syndrome in Severe COVID-19 Cases with Pneumonia and Vascular Thrombosis Could Explain the Post-COVID Syndrome.Biomedicines. 2025 Feb 19;13(2):516. doi: 10.3390/biomedicines13020516. Biomedicines. 2025. PMID: 40002929 Free PMC article.
-
Serum proteomic approach to identifying differentially expressed proteins in effusive feline infectious peritonitis.Sci Rep. 2025 May 29;15(1):18899. doi: 10.1038/s41598-025-03108-2. Sci Rep. 2025. PMID: 40442209 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

