Non-Mammalian Models for Understanding Neurological Defects in RASopathies
- PMID: 38672195
- PMCID: PMC11048513
- DOI: 10.3390/biomedicines12040841
Non-Mammalian Models for Understanding Neurological Defects in RASopathies
Abstract
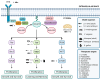
RASopathies, a group of neurodevelopmental congenital disorders stemming from mutations in the RAS/MAPK pathway, present a unique opportunity to delve into the intricacies of complex neurological disorders. Afflicting approximately one in a thousand newborns, RASopathies manifest as abnormalities across multiple organ systems, with a pronounced impact on the central and peripheral nervous system. In the pursuit of understanding RASopathies' neurobiology and establishing phenotype-genotype relationships, in vivo non-mammalian models have emerged as indispensable tools. Species such as Danio rerio, Drosophila melanogaster, Caenorhabditis elegans, Xenopus species and Gallus gallus embryos have proven to be invaluable in shedding light on the intricate pathways implicated in RASopathies. Despite some inherent weaknesses, these genetic models offer distinct advantages over traditional rodent models, providing a holistic perspective on complex genetics, multi-organ involvement, and the interplay among various pathway components, offering insights into the pathophysiological aspects of mutations-driven symptoms. This review underscores the value of investigating the genetic basis of RASopathies for unraveling the underlying mechanisms contributing to broader neurological complexities. It also emphasizes the pivotal role of non-mammalian models in serving as a crucial preliminary step for the development of innovative therapeutic strategies.
Keywords: RAS/MAPK pathway; RASopathies; neurobiology; neurodevelopmental disorders; non-mammalian models; phenotype–genotype relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Johnston J.J., van der Smagt J.J., Rosenfeld J.A., Pagnamenta A.T., Alswaid A., Baker E.H., Blair E., Borck G., Brinkmann J., Craigen W., et al. Autosomal Recessive Noonan Syndrome Associated with Biallelic LZTR1 Variants. Genet. Med. 2018;20:1175–1185. doi: 10.1038/gim.2017.249. - DOI - PMC - PubMed
-
- Motta M., Fasano G., Gredy S., Brinkmann J., Bonnard A.A., Simsek-Kiper P.O., Gulec E.Y., Essaddam L., Utine G.E., Guarnetti Prandi I., et al. SPRED2 Loss-of-Function Causes a Recessive Noonan Syndrome-like Phenotype. Am. J. Hum. Genet. 2021;108:2112–2129. doi: 10.1016/j.ajhg.2021.09.007. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

