Interleukin-6 and Lymphocyte-to-Monocyte Ratio Indices Identify Patients with Intrahepatic Cholangiocarcinoma
- PMID: 38672199
- PMCID: PMC11047984
- DOI: 10.3390/biomedicines12040844
Interleukin-6 and Lymphocyte-to-Monocyte Ratio Indices Identify Patients with Intrahepatic Cholangiocarcinoma
Abstract
Background and aims: Intrahepatic cholangiocarcinoma (iCCA) is a fatal biliary tract cancer with a dismal prognosis due to ineffective diagnostic tools with limited clinical utility. This study investigated peripheral blood indices and cytokine levels to diagnose iCCA.
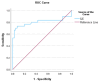
Methods: Blood samples were collected from healthy subjects (n = 48) and patients with advanced-stage iCCA (n = 47) during a phase I and then phase II trial, respectively. Serum cytokines were measured using a flow cytometer. The peripheral blood indices were estimated based on laboratory data. Multi-linear regression analysis was applied, followed by a probability transformation. The cut-off value and model accuracy were determined using the receiver operating curve (ROC) and the area under the curve (AUC).
Results: The interleukin-6 (IL6) and lymphocyte-to-monocyte ratio (LMR) were potential predictors of iCCA [AUC = 0.91 (0.85-0.97) and 0.81 (0.68-0.93); sensitivity = 0.70 and 0.91; specificity = 0.91 and 0.85, respectively]. Patients with IL6 concentrations higher than 11.635 pg/mL (OR = 23.33, p < 0.001) or LMR lower than 7.2 (OR = 58.08, p < 0.001) are at risk of iCCA development. Patients with IL6 levels higher than 21.83 pg/mL, between 15.95 and 21.83 pg/mL, between 8.8 and 15.94 pg/mL, and lower than 8.8 pg/mL were classified as very high-, high-, intermediate-, and low-risk, respectively. Patients with an LMR between 1 and 3.37, 3.38 and 5.76, 5.77 and 7.18, and higher than 7.18 were classified as very high-, high-, intermediate-, and low-risk, respectively.
Conclusions: LMR is recommended for iCCA screening since the estimation is based on a routine laboratory test, which is available in most hospitals.
Keywords: biliary tract cancer; biomarkers; hepatobiliary cancer; intrahepatic cholangiocarcinoma; prognostic predictor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. - DOI - PMC - PubMed
-
- Wongkham S., Silsirivanit A. State of serum markers for detection of cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2012;13:17–27. - PubMed
-
- Kulma I., Panrit L., Plengsuriyakarn T., Chaijaroenkul W., Warathumpitak S., Na-Bangchang K. A randomized placebo-controlled phase I clinical trial to evaluate the immunomodulatory activities of Atractylodes lancea (Thunb) DC. in healthy Thai subjects. BMC Complement. Med. Ther. 2021;21:61. doi: 10.1186/s12906-020-03199-6. - DOI - PMC - PubMed
-
- Na-Bangchang K., Tongsiri N., Plengsuriyakarn T., Sae-heng T., Kongjam P., Kulma I., Worrabannnakorn S., Karbwang J. Phase II-a clinical trial to evaluate safety and efficacy of capsule formulation of the standardized extract of Atractylodes lancea (Thunb.) DC. in patients with advanced stage intrahepatic cholangiocarcinoma. J. Tradit. Complement. Med. 2023 in press .
Grants and funding
LinkOut - more resources
Full Text Sources