L-PRF Secretome from Both Smokers/Nonsmokers Stimulates Angiogenesis and Osteoblast Differentiation In Vitro
- PMID: 38672228
- PMCID: PMC11048676
- DOI: 10.3390/biomedicines12040874
L-PRF Secretome from Both Smokers/Nonsmokers Stimulates Angiogenesis and Osteoblast Differentiation In Vitro
Abstract
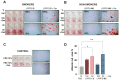
Leukocyte and Platelet-Rich Fibrin (L-PRF) is part of the second generation of platelet-concentrates. L-PRF derived from nonsmokers has been used in surgical procedures, with its beneficial effects in wound healing being proven to stimulate biological activities such as cell proliferation, angiogenesis, and differentiation. Cigarette smoking exerts detrimental effects on tissue healing and is associated with post-surgical complications; however, evidence about the biological effects of L-PRF derived from smokers is limited. This study evaluated the impact of L-PRF secretome (LPRFS) derived from smokers and nonsmokers on angiogenesis and osteoblast differentiation. LPRFS was obtained by submerging L-PRF membranes derived from smokers or nonsmokers in culture media and was used to treat endothelial cells (HUVEC) or SaOs-2 cells. Angiogenesis was evaluated by tubule formation assay, while osteoblast differentiation was observed by alkaline phosphatase and osterix protein levels, as well as in vitro mineralization. LPRFS treatments increased angiogenesis, alkaline phosphatase, and osterix levels. Treatment with 50% of LPRFS derived from smokers and nonsmokers in the presence of osteogenic factors stimulates in vitro mineralization significantly. Nevertheless, differences between LPRFS derived from smokers and nonsmokers were not found. Both LPRFS stimulated angiogenesis and osteoblast differentiation in vitro; however, clinical studies are required to determine the beneficial effect of LPRFS in smokers.
Keywords: angiogenesis; cell differentiation; cigarette smoking; leukocyte- and platelet-rich fibrin; osteoblast; secretome.
Conflict of interest statement
The authors declare no conflicts of interest. The authors confirm that no tobacco company funded this work.
Figures



References
-
- Quirynen M., Siawasch S.A.M., Temmerman A., Cortellini S., Dhondt R., Teughels W., Castro A.B. Do Autologous Platelet Concentrates (APCs) Have a Role in Intra-oral Bone Regeneration? A Critical Review of Clinical Guidelines on Decision-making Process. Periodontology 2000. 2023;93:254–269. doi: 10.1111/prd.12526. - DOI - PubMed
-
- Avila-Ortiz G., Ambruster J., Barootchi S., Chambrone L., Chen C., Dixon D.R., Geisinger M.L., Giannobile W.V., Goss K., Gunsolley J.C., et al. American Academy of Periodontology Best Evidence Consensus Statement on the Use of Biologics in Clinical Practice. J. Periodontol. 2022;93:1763–1770. doi: 10.1002/JPER.22-0361. - DOI - PMC - PubMed
-
- Castro A.B., Meschi N., Temmerman A., Pinto N., Lambrechts P., Teughels W., Quirynen M. Regenerative Potential of Leucocyte- and Platelet-Rich Fibrin. Part B: Sinus Floor Elevation, Alveolar Ridge Preservation and Implant Therapy. A Systematic Review. J. Clin. Periodontol. 2017;44:225–234. doi: 10.1111/jcpe.12658. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

