Verapamil Attenuates the Severity of Tendinopathy by Mitigating Mitochondrial Dysfunction through the Activation of the Nrf2/HO-1 Pathway
- PMID: 38672259
- PMCID: PMC11048132
- DOI: 10.3390/biomedicines12040904
Verapamil Attenuates the Severity of Tendinopathy by Mitigating Mitochondrial Dysfunction through the Activation of the Nrf2/HO-1 Pathway
Abstract
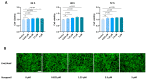
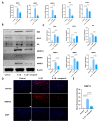
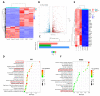
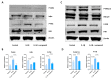
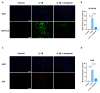
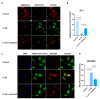
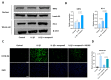
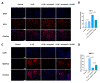
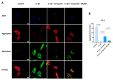
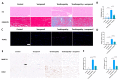
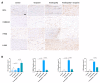
Tendinopathy is a prevalent condition in orthopedics patients, exerting a profound impact on tendon functionality. However, its underlying mechanism remains elusive and the efficacy of pharmacological interventions continues to be suboptimal. Verapamil is a clinically used medicine with anti-inflammation and antioxidant functions. This investigation aimed to elucidate the impact of verapamil in tendinopathy and the underlying mechanisms through which verapamil ameliorates the severity of tendinopathy. In in vitro experiments, primary tenocytes were exposed to interleukin-1 beta (IL-1β) along with verapamil at a concentration of 5 μM. In addition, an in vivo rat tendinopathy model was induced through the localized injection of collagenase into the Achilles tendons of rats, and verapamil was injected into these tendons at a concentration of 5 μM. The in vitro findings highlighted the remarkable ability of verapamil to attenuate extracellular matrix degradation and apoptosis triggered by inflammation in tenocytes stimulated by IL-1β. Furthermore, verapamil was observed to significantly suppress the inflammation-related MAPK/NFκB pathway. Subsequent investigations revealed that verapamil exerts a remediating effect on mitochondrial dysfunction, which was achieved through activation of the Nrf2/HO-1 pathway. Nevertheless, the protective effect of verapamil was nullified with the utilization of the Nrf2 inhibitor ML385. In summary, the in vivo and in vitro results indicate that the administration of verapamil profoundly mitigates the severity of tendinopathy through suppression of inflammation and activation of the Nrf2/HO-1 pathway. These findings suggest that verapamil is a promising therapeutic agent for the treatment of tendinopathy, deserving further and expanded research.
Keywords: Nrf2/HO-1 axis; ROS; mitochondrial dysfunction; tendinopathy; verapamil.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mitochondrial transfer from bone mesenchymal stem cells protects against tendinopathy both in vitro and in vivo.Stem Cell Res Ther. 2023 Apr 26;14(1):104. doi: 10.1186/s13287-023-03329-0. Stem Cell Res Ther. 2023. PMID: 37101277 Free PMC article.
-
Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models.Biochem Pharmacol. 2020 May;175:113919. doi: 10.1016/j.bcp.2020.113919. Epub 2020 Mar 17. Biochem Pharmacol. 2020. PMID: 32194057
-
Fullerenol inhibits tendinopathy by alleviating inflammation.Front Bioeng Biotechnol. 2023 Mar 30;11:1171360. doi: 10.3389/fbioe.2023.1171360. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37064249 Free PMC article.
-
Inhibition of CD44 induces apoptosis, inflammation, and matrix metalloproteinase expression in tendinopathy.J Biol Chem. 2019 Dec 27;294(52):20177-20184. doi: 10.1074/jbc.RA119.009675. Epub 2019 Nov 15. J Biol Chem. 2019. PMID: 31732563 Free PMC article.
-
Mitochondrial destabilization in tendinopathy and potential therapeutic strategies.J Orthop Translat. 2024 Oct 3;49:49-61. doi: 10.1016/j.jot.2024.09.003. eCollection 2024 Nov. J Orthop Translat. 2024. PMID: 39430132 Free PMC article. Review.
Cited by
-
Ectopic calcifications in the musculoskeletal field: the basis for preventive and curative pharmacological strategies.Clin Rheumatol. 2025 Mar;44(3):869-886. doi: 10.1007/s10067-025-07335-w. Epub 2025 Jan 24. Clin Rheumatol. 2025. PMID: 39853559 Review.
-
Ganoderma lucidum Polysaccharide Peptide Alleviates Cyclophosphamide-Induced Male Reproductive Injury by Reducing Oxidative Stress and Apoptosis.Biomedicines. 2024 Jul 23;12(8):1632. doi: 10.3390/biomedicines12081632. Biomedicines. 2024. PMID: 39200097 Free PMC article.
References
-
- Wang J.H., Jia F., Yang G., Yang S., Campbell B.H., Stone D., Woo S.L. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: A novel in vitro model study. Connect. Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

