The Role of Cdo1 in Ferroptosis and Apoptosis in Cancer
- PMID: 38672271
- PMCID: PMC11047957
- DOI: 10.3390/biomedicines12040918
The Role of Cdo1 in Ferroptosis and Apoptosis in Cancer
Abstract
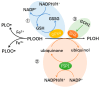
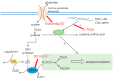
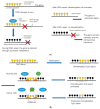
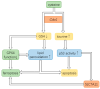
Cysteine dioxygenase type 1 (Cdo1) is a tumor suppressor gene. It regulates the metabolism of cysteine, thereby influencing the cellular antioxidative capacity. This function puts Cdo1 in a prominent position to promote ferroptosis and apoptosis. Cdo1 promotes ferroptosis mainly by decreasing the amounts of antioxidants, leading to autoperoxidation of the cell membrane through Fenton reaction. Cdo1 promotes apoptosis mainly through the product of cysteine metabolism, taurine, and low level of antioxidants. Many cancers exhibit altered function of Cdo1, underscoring its crucial role in cancer cell survival. Genetic and epigenetic alterations have been found, with methylation of Cdo1 promoter as the most common mutation. The fact that no cancer was found to be caused by altered Cdo1 function alone indicates that the tumor suppressor role of Cdo1 is mild. By compiling the current knowledge about apoptosis, ferroptosis, and the role of Cdo1, this review suggests possibilities for how the mild anticancer role of Cdo1 could be harnessed in new cancer therapies. Here, developing drugs targeting Cdo1 is considered meaningful in neoadjuvant therapies, for example, helping against the development of anti-cancer drug resistance in tumor cells.
Keywords: Cdo1; apoptosis; cancer; cysteine; ferroptosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Cysteine dioxygenase type 1 (CDO1): Its functional role in physiological and pathophysiological processes.Genes Dis. 2022 Feb 17;10(3):877-890. doi: 10.1016/j.gendis.2021.12.023. eCollection 2023 May. Genes Dis. 2022. PMID: 37396540 Free PMC article. Review.
-
Targeted demethylation of the CDO1 promoter based on CRISPR system inhibits the malignant potential of breast cancer cells.Clin Transl Med. 2023 Sep;13(9):e1423. doi: 10.1002/ctm2.1423. Clin Transl Med. 2023. PMID: 37740473 Free PMC article.
-
Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers.PLoS One. 2012;7(9):e44951. doi: 10.1371/journal.pone.0044951. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23028699 Free PMC article.
-
Methylated cysteine dioxygenase-1 gene promoter in the serum is a potential biomarker for hepatitis B virus-related hepatocellular carcinoma.Tohoku J Exp Med. 2014 Mar;232(3):187-94. doi: 10.1620/tjem.232.187. Tohoku J Exp Med. 2014. PMID: 24646840
-
[Molecular mechanism of CDO1 regulating common metabolic diseases].Sheng Li Xue Bao. 2024 Aug 25;76(4):576-586. Sheng Li Xue Bao. 2024. PMID: 39192790 Review. Chinese.
Cited by
-
Dynamics of the immune microenvironment and immune cell PANoptosis in colorectal cancer: recent advances and insights.Front Immunol. 2024 Nov 29;15:1502257. doi: 10.3389/fimmu.2024.1502257. eCollection 2024. Front Immunol. 2024. PMID: 39676861 Free PMC article. Review.
-
Ferroptosis-mediated immune responses in osteoporosis.J Orthop Translat. 2025 Apr 12;52:116-125. doi: 10.1016/j.jot.2025.03.011. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40271049 Free PMC article. Review.
-
Construction and validation of a nomogram model for predicting peritoneal metastasis in gastric cancer based on ferroptosis-relate genes and clinicopathological features.J Gastrointest Oncol. 2025 Feb 28;16(1):264-280. doi: 10.21037/jgo-24-670. Epub 2025 Feb 26. J Gastrointest Oncol. 2025. PMID: 40115916 Free PMC article.
-
Research Progress of DNA Methylation Markers for Endometrial Carcinoma Diagnosis.J Cancer. 2025 Jan 1;16(3):812-820. doi: 10.7150/jca.104214. eCollection 2025. J Cancer. 2025. PMID: 39781343 Free PMC article. Review.
-
Exploring the Role of CDO1 in Breast Cancer: Insights into Tumor Biology and Therapeutic Potential.Ann Surg Oncol. 2025 Aug 5. doi: 10.1245/s10434-025-17944-z. Online ahead of print. Ann Surg Oncol. 2025. PMID: 40762777
References
Publication types
LinkOut - more resources
Full Text Sources

