Applications of Hydrogels in Osteoarthritis Treatment
- PMID: 38672277
- PMCID: PMC11048369
- DOI: 10.3390/biomedicines12040923
Applications of Hydrogels in Osteoarthritis Treatment
Abstract
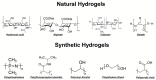
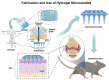
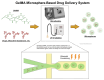
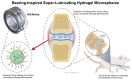
This review critically evaluates advancements in multifunctional hydrogels, particularly focusing on their applications in osteoarthritis (OA) therapy. As research evolves from traditional natural materials, there is a significant shift towards synthetic and composite hydrogels, known for their superior mechanical properties and enhanced biodegradability. This review spotlights novel applications such as injectable hydrogels, microneedle technology, and responsive hydrogels, which have revolutionized OA treatment through targeted and efficient therapeutic delivery. Moreover, it discusses innovative hydrogel materials, including protein-based and superlubricating hydrogels, for their potential to reduce joint friction and inflammation. The integration of bioactive compounds within hydrogels to augment therapeutic efficacy is also examined. Furthermore, the review anticipates continued technological advancements and a deeper understanding of hydrogel-based OA therapies. It emphasizes the potential of hydrogels to provide tailored, minimally invasive treatments, thus highlighting their critical role in advancing the dynamic field of biomaterial science for OA management.
Keywords: Biomaterials; Drug delivery; Hydrogel; Interdisciplinary therapy; Osteoarthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration.Acta Biomater. 2021 Dec;136:1-36. doi: 10.1016/j.actbio.2021.09.034. Epub 2021 Sep 23. Acta Biomater. 2021. PMID: 34562661 Review.
-
Hydrogel-Based Biomaterials: A Patent Landscape on Innovation Trends and Patterns.Gels. 2025 Mar 20;11(3):216. doi: 10.3390/gels11030216. Gels. 2025. PMID: 40136921 Free PMC article. Review.
-
Injectable Hydrogel Technologies for Bone Disease Treatment.ACS Appl Bio Mater. 2025 Apr 21;8(4):2691-2715. doi: 10.1021/acsabm.4c01968. Epub 2025 Apr 7. ACS Appl Bio Mater. 2025. PMID: 40193334 Review.
-
Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering.Cells. 2020 Feb 12;9(2):419. doi: 10.3390/cells9020419. Cells. 2020. PMID: 32059502 Free PMC article. Review.
-
Harnessing the power of biological macromolecules in hydrogels for controlled drug release in the central nervous system: A review.Int J Biol Macromol. 2024 Jan;254(Pt 1):127708. doi: 10.1016/j.ijbiomac.2023.127708. Epub 2023 Nov 1. Int J Biol Macromol. 2024. PMID: 37923043 Review.
Cited by
-
Lubricant Strategies in Osteoarthritis Treatment: Transitioning from Natural Lubricants to Drug Delivery Particles with Lubricant Properties.J Xenobiot. 2024 Sep 19;14(3):1268-1292. doi: 10.3390/jox14030072. J Xenobiot. 2024. PMID: 39311151 Free PMC article. Review.
-
Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression.Int J Mol Sci. 2024 Nov 11;25(22):12082. doi: 10.3390/ijms252212082. Int J Mol Sci. 2024. PMID: 39596150 Free PMC article. Review.
-
Association of autoimmune diseases with the occurrence of osteoarthritis: a gene expression and Mendelian randomization study.Front Med (Lausanne). 2024 Sep 5;11:1435312. doi: 10.3389/fmed.2024.1435312. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39301493 Free PMC article.
-
Polymeric Microneedle Drug Delivery Systems: Mechanisms of Treatment, Material Properties, and Clinical Applications-A Comprehensive Review.Polymers (Basel). 2024 Sep 11;16(18):2568. doi: 10.3390/polym16182568. Polymers (Basel). 2024. PMID: 39339032 Free PMC article. Review.
-
Recent Evidence for Orthobiologics Combined with Hydrogels for Joint Tissue Regeneration: Focus on Osteoarthritis.Gels. 2025 Jul 17;11(7):551. doi: 10.3390/gels11070551. Gels. 2025. PMID: 40710712 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

