Periparturient Mineral Metabolism: Implications to Health and Productivity
- PMID: 38672379
- PMCID: PMC11047658
- DOI: 10.3390/ani14081232
Periparturient Mineral Metabolism: Implications to Health and Productivity
Abstract
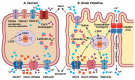
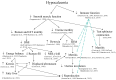
Mineral metabolism, in particular Ca, and to a lesser extent phosphorus (P) and magnesium (Mg), is altered with the onset of lactation because of extensive irreversible loss to synthesize colostrum and milk. The transient reduction in the concentration of Ca in blood, particularly when it lasts days, increases the risk of mineral-related disorders such as hypocalcemia and, to a lesser extent, hypophosphatemia. Although the incidence of clinical hypocalcemia can be reduced by prepartum dietary interventions, subclinical hypocalcemia remains prevalent, affecting up to 60% of the dairy cows in the first 3 d postpartum. More importantly, strong associations exist between hypocalcemia and increased susceptibility to other peripartum diseases and impaired reproductive performance. Mechanistic experiments have demonstrated the role of Ca on innate immune response in dairy cows, which presumably predisposes them to other diseases. Hypocalcemia is not related to inadequate Ca intake as prepartum diets marginal to deficient in Ca reduce the risk of the disease. Therefore, the understanding of how Ca homeostasis is regulated, in particular how calciotropic hormones such as parathyroid hormone and 1,25-dihydroxyvitamin D3, affect blood Ca concentrations, gastrointestinal Ca absorption, bone remodeling, and renal excretion of Ca become critical to develop novel strategies to prevent mineral imbalances either by nutritional or pharmacological interventions. A common method to reduce the risk of hypocalcemia is the manipulation of the prepartum dietary cation-anion difference. Feeding acidogenic diets not only improves Ca homeostasis and reduces hypocalcemia, but also reduces the risk of uterine diseases and improves productive performance. Feeding diets that induce a negative Ca balance in the last weeks of gestation also reduce the risk of clinical hypocalcemia, and recent work shows that the incorporation of mineral sequestering agents, presumably by reducing the absorption of P and Ca prepartum, increases blood Ca at calving, although benefits to production and health remain to be shown. Alternative strategies to minimize subclinical hypocalcemia with the use of vitamin D metabolites either fed prepartum or as a pharmacological agent administered immediately after calving have shown promising results in reducing hypocalcemia and altering immune cell function, which might prove efficacious to prevent diseases in early lactation. This review summarizes the current understanding of Ca homeostasis around parturition, the limited knowledge of the exact mechanisms for gastrointestinal Ca absorption in bovine, the implications of hypocalcemia on the health of dairy cows, and discusses the methods to minimize the risk of hypocalcemia and their impacts on productive performance and health in dairy cows.
Keywords: calcium; dairy cow; dietary cation-anion difference; mineral.
Conflict of interest statement
Ian J. Lean is a partner owner of Scibus, Camden, Australia. The authors declare that the contents of his review article were prepared in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures

References
-
- Lopera C., Zimpel R., Vieira-Neto A., Lopes F.R., Ortiz W., Poindexter M., Faria B.N., Gambarini M.L., Block E., Nelson C.D., et al. Effects of level of dietary cation-anion difference and duration of prepartum feeding on performance and metabolism of dairy cows. J. Dairy Sci. 2018;101:7907–7929. doi: 10.3168/jds.2018-14580. - DOI - PubMed
-
- Rodney R.M., Martinez N., Block E., Hernandez L.L., Celi P., Nelson C.D., Santos J.E.P., Lean I.J. Effects of prepartum dietary cation-anion difference and source of vitamin D in dairy cows: Vitamin D, mineral and bone metabolism. J. Dairy Sci. 2018;101:2519–2543. doi: 10.3168/jds.2017-13737. - DOI - PubMed
-
- NRC . Nutrient Requirements of Dairy Cattle. 7th ed. National Academies Press; Washington, DC, USA: 2001. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

