In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa-Implementation of Three Gallium-Based Complexes in the "Trojan Horse" Antibacterial Strategy
- PMID: 38672503
- PMCID: PMC11048449
- DOI: 10.3390/biom14040487
In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa-Implementation of Three Gallium-Based Complexes in the "Trojan Horse" Antibacterial Strategy
Abstract
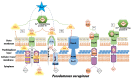
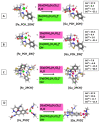
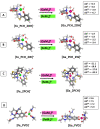
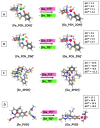
The emergence of multidrug-resistant (MDR) microorganisms combined with the ever-draining antibiotic pipeline poses a disturbing and immensely growing public health challenge that requires a multidisciplinary approach and the application of novel therapies aimed at unconventional targets and/or applying innovative drug formulations. Hence, bacterial iron acquisition systems and bacterial Fe2+/3+-containing enzymes have been identified as a plausible target of great potential. The intriguing "Trojan horse" approach deprives microorganisms from the essential iron. Recently, gallium's potential in medicine as an iron mimicry species has attracted vast attention. Different Ga3+ formulations exhibit diverse effects upon entering the cell and thus supposedly have multiple targets. The aim of the current study is to specifically distinguish characteristics of great significance in regard to the initial gallium-based complex, allowing the alien cation to effectively compete with the native ferric ion for binding the siderophores pyochelin and pyoverdine secreted by the bacterium P. aeruginosa. Therefore, three gallium-based formulations were taken into consideration: the first-generation gallium nitrate, Ga(NO3)3, metabolized to Ga3+-hydrated forms, the second-generation gallium maltolate (tris(3-hydroxy-2-methyl-4-pyronato)gallium), and the experimentally proven Ga carrier in the bloodstream-the protein transferrin. We employed a reliable in silico approach based on DFT computations in order to understand the underlying biochemical processes that govern the Ga3+/Fe3+ rivalry for binding the two bacterial siderophores.
Keywords: DFT; ESKAPE; Ga3+/Fe3+ competition; Pseudomonas aeruginosa; maltolate; pyochelin; pyoverdine; siderophore; transferrin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores.Inorg Chem. 2020 May 4;59(9):6242-6254. doi: 10.1021/acs.inorgchem.0c00367. Epub 2020 Apr 14. Inorg Chem. 2020. PMID: 32286066
-
Understanding the biomimetic properties of gallium in Pseudomonas aeruginosa: an XAS and XPS study.Dalton Trans. 2017 May 30;46(21):7082-7091. doi: 10.1039/c7dt00651a. Dalton Trans. 2017. PMID: 28524209
-
Implementation of Three Gallium-Based Complexes in the "Trojan Horse" Antibacterial Strategy against A. baumannii: A DFT Approach.Inorg Chem. 2024 Aug 19;63(33):15409-15420. doi: 10.1021/acs.inorgchem.4c02411. Epub 2024 Aug 8. Inorg Chem. 2024. PMID: 39116415
-
Roles of Pseudomonas aeruginosa siderophores in interaction with prokaryotic and eukaryotic organisms.Res Microbiol. 2024 Sep-Oct;175(7):104211. doi: 10.1016/j.resmic.2024.104211. Epub 2024 May 9. Res Microbiol. 2024. PMID: 38734157 Review.
-
Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species.Biometals. 2011 Jun;24(3):513-22. doi: 10.1007/s10534-010-9399-9. Epub 2010 Dec 25. Biometals. 2011. PMID: 21188474 Review.
Cited by
-
Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria.Molecules. 2025 Jul 23;30(15):3072. doi: 10.3390/molecules30153072. Molecules. 2025. PMID: 40807247 Free PMC article.
-
Wound repair and immune function in the Pseudomonas infected CF lung: before and after highly effective modulator therapy.Front Cell Infect Microbiol. 2025 Apr 28;15:1566495. doi: 10.3389/fcimb.2025.1566495. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40357395 Free PMC article. Review.
References
-
- World Health Organization List of Antibiotic Resistant Priority Pathogens. [(accessed on 23 January 2024)]. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-n....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

