Mitochondrial Transplantation's Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging
- PMID: 38672509
- PMCID: PMC11048484
- DOI: 10.3390/biom14040493
Mitochondrial Transplantation's Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging
Abstract
Background: Mitochondria are the 'powerhouses of cells' and progressive mitochondrial dysfunction is a hallmark of aging in skeletal muscle. Although different forms of exercise modality appear to be beneficial to attenuate aging-induced mitochondrial dysfunction, it presupposes that the individual has a requisite level of mobility. Moreover, non-exercise alternatives (i.e., nutraceuticals or pharmacological agents) to improve skeletal muscle bioenergetics require time to be effective in the target tissue and have another limitation in that they act systemically and not locally where needed. Mitochondrial transplantation represents a novel directed therapy designed to enhance energy production of tissues impacted by defective mitochondria. To date, no studies have used mitochondrial transplantation as an intervention to attenuate aging-induced skeletal muscle mitochondrial dysfunction. The purpose of this investigation, therefore, was to determine whether mitochondrial transplantation can enhance skeletal muscle bioenergetics in an aging rodent model. We hypothesized that mitochondrial transplantation would result in sustained skeletal muscle bioenergetics leading to improved functional capacity.
Methods: Fifteen female mice (24 months old) were randomized into two groups (placebo or mitochondrial transplantation). Isolated mitochondria from a donor mouse of the same sex and age were transplanted into the hindlimb muscles of recipient mice (quadriceps femoris, tibialis anterior, and gastrocnemius complex).
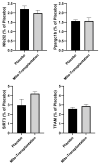
Results: The results indicated significant increases (ranging between ~36% and ~65%) in basal cytochrome c oxidase and citrate synthase activity as well as ATP levels in mice receiving mitochondrial transplantation relative to the placebo. Moreover, there were significant increases (approx. two-fold) in protein expression of mitochondrial markers in both glycolytic and oxidative muscles. These enhancements in the muscle translated to significant improvements in exercise tolerance.
Conclusions: This study provides initial evidence showing how mitochondrial transplantation can promote skeletal muscle bioenergetics in an aging rodent model.
Keywords: endurance; energy production; exercise physiology.
Conflict of interest statement
McCully has patents pending for the isolation and usage of mitochondria and is a Co-founder and Board Member of Cellvie Scientific.
Figures




Similar articles
-
Age-related changes of skeletal muscle metabolic response to contraction are also sex-dependent.J Physiol. 2025 Jan;603(1):69-86. doi: 10.1113/JP285124. Epub 2023 Sep 23. J Physiol. 2025. PMID: 37742081 Free PMC article.
-
Mitochondrial energetics is impaired in vivo in aged skeletal muscle.Aging Cell. 2014 Feb;13(1):39-48. doi: 10.1111/acel.12147. Epub 2013 Sep 19. Aging Cell. 2014. PMID: 23919652 Free PMC article.
-
Age-related decline in murine heart and skeletal muscle performance is attenuated by reduced Ahnak1 expression.J Cachexia Sarcopenia Muscle. 2021 Oct;12(5):1249-1265. doi: 10.1002/jcsm.12749. Epub 2021 Jul 1. J Cachexia Sarcopenia Muscle. 2021. PMID: 34212535 Free PMC article.
-
Skeletal muscle bioenergetics in aging and heart failure.Heart Fail Rev. 2017 Mar;22(2):167-178. doi: 10.1007/s10741-016-9586-z. Heart Fail Rev. 2017. PMID: 27815651 Free PMC article. Review.
-
Skeletal muscle mitochondrial energetic efficiency and aging.Int J Mol Sci. 2015 May 11;16(5):10674-85. doi: 10.3390/ijms160510674. Int J Mol Sci. 2015. PMID: 25970752 Free PMC article. Review.
Cited by
-
Mitochondrial dysfunction in the regulation of aging and aging-related diseases.Cell Commun Signal. 2025 Jun 19;23(1):290. doi: 10.1186/s12964-025-02308-7. Cell Commun Signal. 2025. PMID: 40537801 Free PMC article. Review.
-
Therapeutic transplantation of mitochondria and Extracellular Vesicles: Mechanistic insights into mitochondria bioenergetics, redox signaling, and organelle dynamics in preclinical models.Free Radic Biol Med. 2025 Oct;238:473-495. doi: 10.1016/j.freeradbiomed.2025.06.040. Epub 2025 Jun 24. Free Radic Biol Med. 2025. PMID: 40570985 Free PMC article. Review.
-
Mechanism and prospects of mitochondrial transplantation for spinal cord injury treatment.Stem Cell Res Ther. 2024 Nov 28;15(1):457. doi: 10.1186/s13287-024-04077-5. Stem Cell Res Ther. 2024. PMID: 39609871 Free PMC article. Review.
References
-
- Lemes I.R., Sui X., Fritz S.L., Beattie P.F., Lavie C.J., Turi-Lynch B.C., Blair S.N. Cardiorespiratory Fitness and Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality in Men with Musculoskeletal Conditions. J. Phys. Act. Health. 2019;16:134–140. doi: 10.1123/jpah.2017-0644. - DOI - PubMed
-
- Roshanravan B., Liu S.Z., Ali A.S., Shankland E.G., Goss C., Amory J.K., Robertson H.T., Marcinek D.J., Conley K.E. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PLoS ONE. 2021;16:e0253849. doi: 10.1371/journal.pone.0253849. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

