Bevacizumab Treatment for Patients with NF2-Related Schwannomatosis: A Single Center Experience
- PMID: 38672561
- PMCID: PMC11047890
- DOI: 10.3390/cancers16081479
Bevacizumab Treatment for Patients with NF2-Related Schwannomatosis: A Single Center Experience
Abstract
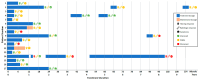
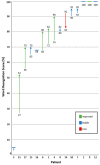
(1) Background: NF2-related schwannomatosis, characterized by the development of bilateral vestibular schwannomas, often necessitates varied treatment approaches. Bevacizumab, though widely utilized, demonstrates variable effectiveness on hearing and tumor growth. At the same time, (serious) adverse events have been frequently reported. (2) Methods: A single center retrospective study was conducted, on NF2-related schwannomatosis patients treated with bevacizumab from 2013 to 2023, with the aim to assess treatment-related and clinical outcomes. Outcomes of interest comprised hearing, radiologic response, symptoms, and adverse events. (3) Results: Seventeen patients received 7.5 mg/kg bevacizumab for 7.1 months. Following treatment, 40% of the patients experienced hearing improvement, 53%, stable hearing, and 7%, hearing loss. Vestibular schwannoma regression occurred in 31%, and 69% remained stable. Further symptomatic improvement was reported by 41%, stable symptoms by 47%, and worsened symptoms by 12%. Treatment discontinuation due to adverse events was observed in 29% of cases. Hypertension (82%) and fatigue (29%) were most frequently reported, with no occurrences of grade 4/5 toxicities. (4) Conclusion: Supporting previous studies, bevacizumab demonstrated positive effects on hearing, tumor control, and symptoms in NF2-related schwannomatosis, albeit with common adverse events. Therefore, careful consideration of an appropriate management strategy is warranted.
Keywords: acoustic neuroma; adverse events; bevacizumab; cerebellopontine angle tumor; hearing; neurofibromatosis type 2; schwannomatosis; tumor response; vestibular schwannoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Bevacizumab for Vestibular Schwannomas in Neurofibromatosis Type 2: A Systematic Review of Tumor Control and Hearing Preservation.J Clin Med. 2024 Dec 9;13(23):7488. doi: 10.3390/jcm13237488. J Clin Med. 2024. PMID: 39685944 Free PMC article. Review.
-
Efficacy and Toxicity of Bevacizumab in Children with NF2-Related Schwannomatosis: A Systematic Review.Cancers (Basel). 2025 Feb 4;17(3):519. doi: 10.3390/cancers17030519. Cancers (Basel). 2025. PMID: 39941885 Free PMC article. Review.
-
Multicenter, prospective, phase II study of maintenance bevacizumab for children and adults with NF2-related schwannomatosis and progressive vestibular schwannoma.Neuro Oncol. 2023 Aug 3;25(8):1498-1506. doi: 10.1093/neuonc/noad066. Neuro Oncol. 2023. PMID: 37010875 Free PMC article. Clinical Trial.
-
Proton Radiotherapy for Vestibular Schwannomas in Patients with NF2-Related Schwannomatosis: A Case Series.Curr Oncol. 2023 Mar 20;30(3):3473-3483. doi: 10.3390/curroncol30030263. Curr Oncol. 2023. PMID: 36975476 Free PMC article.
-
Withdrawal of bevacizumab is associated with rebound growth of vestibular schwannomas in neurofibromatosis type 2-related schwannomatosis patients.Neurooncol Adv. 2023 Sep 22;5(1):vdad123. doi: 10.1093/noajnl/vdad123. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37841698 Free PMC article.
Cited by
-
Management Strategies of Neurofibromatosis Type 2 in Pediatric Patients : Challenges and Emerging Therapies.J Korean Neurosurg Soc. 2025 May;68(3):278-285. doi: 10.3340/jkns.2024.0237. Epub 2025 Feb 24. J Korean Neurosurg Soc. 2025. PMID: 39988763 Free PMC article.
-
Bevacizumab for Vestibular Schwannomas in Neurofibromatosis Type 2: A Systematic Review of Tumor Control and Hearing Preservation.J Clin Med. 2024 Dec 9;13(23):7488. doi: 10.3390/jcm13237488. J Clin Med. 2024. PMID: 39685944 Free PMC article. Review.
-
Efficacy and Toxicity of Bevacizumab in Children with NF2-Related Schwannomatosis: A Systematic Review.Cancers (Basel). 2025 Feb 4;17(3):519. doi: 10.3390/cancers17030519. Cancers (Basel). 2025. PMID: 39941885 Free PMC article. Review.
-
Genetic Basis and Clinical Management of Schwannomatosis.J Korean Neurosurg Soc. 2025 May;68(3):286-293. doi: 10.3340/jkns.2025.0001. Epub 2025 Mar 6. J Korean Neurosurg Soc. 2025. PMID: 40049215 Free PMC article.
-
From bench to bedside: Novel TGFβR1 inhibitor shows promise in neurofibromatosis treatment.Neuro Oncol. 2025 Jun 21;27(5):1256-1257. doi: 10.1093/neuonc/noaf054. Neuro Oncol. 2025. PMID: 40207551 No abstract available.
References
-
- Evans D. Chapter 5—Neurofibromatosis type 2. In: Islam M.P., Roach E.S., editors. Neurocutaneous Syndromes. Vol. 132. Elsevier; Amsterdam, The Netherlands: 2015. pp. 87–96.
-
- Blakeley J.O., Evans D.G., Adler J., Brackmann D., Chen R., Ferner R.E., Hanemann C.O., Harris G., Huson S.M., Jacob A., et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am. J. Med. Genet. A. 2012;158A:24–41. doi: 10.1002/ajmg.a.34359. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

