Availability and Access to Orphan Drugs for Rare Cancers in Bulgaria: Analysis of Delays and Public Expenditures
- PMID: 38672571
- PMCID: PMC11048562
- DOI: 10.3390/cancers16081489
Availability and Access to Orphan Drugs for Rare Cancers in Bulgaria: Analysis of Delays and Public Expenditures
Abstract
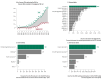
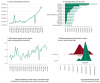
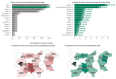
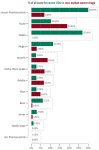
Rare cancers are defined by an annual incidence of fewer than 6 per 100,000. Bearing similarities to rare diseases, they are associated with substantial health inequalities due to diagnostic complexity and delayed access to innovative therapies. This situation is further aggravated in Southeastern European countries like Bulgaria, where limited public resources and expertise underscore the need for additional policy and translational research on rare cancers. This study aimed to explore the availability and access to orphan drugs for rare cancers in Bulgaria for the period of 2020-2023. We cross-compared data from both the European Union and national public sources to evaluate the number of available and accessible orphan drugs for rare cancers, the delay from market authorization to reimbursement, the dynamics of public expenditures, and regional disparities in access across the country. We juxtaposed the main characteristics of oncological and non-oncological orphan drugs as well. Only 15 out of 50 oncological orphan drugs that were authorized by the European Medicine Agency were accessible for rare cancer patients in Bulgaria. The median delay between market authorization and inclusion in the Bulgarian Positive Drug List was 760 days. The total expenditures for all orphan drugs for rare cancers amounted to EUR 74,353,493 from 2020 to 2023. The budgetary impact of this group rose from 0.24% to 3.77% of total public medicinal product expenditures for the study period. Rare cancer patients represent a vulnerable population that often faces limited to no access to treatment. We call for targeted European and national policies to address this major inequality.
Keywords: Bulgaria; cancer costs; health inequalities; orphan drugs; rare cancers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- JARC—Joint Action on Rare Cancers Rare Cancer Agenda—2030. Rare Cancers Europe 2022. [(accessed on 3 January 2024)]. Available online: https://www.esmo.org/policy/rare-cancers-working-group/rare-cancers-in-e....
-
- European Society for Medical Oncology—ESMO [(accessed on 3 January 2024)]. Available online: https://www.esmo.org/
-
- de la Paz M.P., Taruscio D., Groft S.C., editors. Rare Diseases Epidemiology: Update and Overview|SpringerLink. Springer; Dordrecht, The Netherlands: 2010. [(accessed on 3 January 2024)]. Advances in Experimental Medicine and Biology 686. Available online: https://link.springer.com/book/10.1007/978-3-319-67144-4. - DOI
-
- Law of 10 November 2021/No. 175. Provisions for the Treatment of Rare Diseases and the Support of Research and Production of Orphan Drugs. (21G00189) [LEGGE 10 November 2021/n. 175. Disposizioni Per la Cura Delle Malattie Rare e Per il Sostegno Della Ricerca e Della Produzione Dei Farmaci Orfani. (21G00189)] 2021. [(accessed on 3 January 2024)]. Available online: https://www.gazzettaufficiale.it/eli/gu/2021/11/27/283/sg/pdf.
-
- Commission Staff Working Document EU Missions Two Years on: An Assessment of Progress in Shaping the Future We Want and Reporting on the Review of Mission Areas and Areas for Institutionalised Partnerships Based on Articles 185 and 187 TFEU Accompanying the Document Communication From the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions EU Missions Two Years on: Assessment of Progress and Way Forward 2023. [(accessed on 3 January 2024)]. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:52023SC0260.
LinkOut - more resources
Full Text Sources
Miscellaneous

