CALR but Not JAK2 Mutations Are Associated with an Overexpression of Retinoid X Receptor Alpha in Essential Thrombocythemia
- PMID: 38672593
- PMCID: PMC11048154
- DOI: 10.3390/cancers16081511
CALR but Not JAK2 Mutations Are Associated with an Overexpression of Retinoid X Receptor Alpha in Essential Thrombocythemia
Abstract
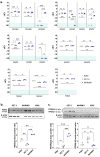
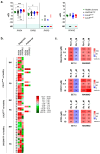
Essential thrombocythemia (ET) is a blood cancer caused by mutations in JAK2 and CALR. It is widely recognized that both mutations lead to the constitutive activation of JAK2/STAT signaling, although other JAK/STAT-independent pathogenic mechanisms triggered by these alterations have also been described in ET. In an attempt to study JAK2/STAT-independent mechanisms derived from CALR mutations, our research group created a C. elegans model with patient-like mutations in calreticulin that lacks JAK counterparts. The introduction of patient-like mutations in the calreticulin of C. elegans leads to an increase in the transcriptional expression of nhr-2, independently of JAK2/STAT activation. In the present study, we aim to verify if this mechanism is conserved in patients with ET harboring CALR mutations. To do so, we evaluated the expression of potential orthologs of nhr-2 in human cell lines of interest for the study, as well as in bone marrow (BM) or peripheral blood (PB) mononuclear cells from patients with CALR or JAK2 mutations. The results revealed that this mechanism is conserved in CALR-mutated ET patients, since CALR, but not JAK2 mutations, were associated with an overexpression of RXRA in patients with ET. The use of drugs targeting the activation or blockade of this target in the analyzed cell lines did not result in changes in cell viability. However, RXRA might be relevant in the disease, pointing to the need for future research testing retinoids and other drugs targeting RXRα for the treatment of ET patients.
Keywords: CALR; ET; RXRA; myeloproliferative neoplasms.
Conflict of interest statement
A.G.-H., C.H., M.J.L. and J.L.V. declare no conflicts of interest. M.J.C. declares honoraria for lectures from and membership on advisory boards with Janssen, Jazz Pharmaceuticals, Astellas, Novartis, Amgen, and Bristol-Myers Squibb-Celgene.
Figures
References
-
- Uozumi K., Otsuka M., Ohno N., Moriyama T., Suzuki S., Shimotakahara S., Matsumura I., Hanada S., Arima T. Establishment and characterization of a new human megakaryoblastic cell line (SET-2) that spontaneously matures to megakaryocytes and produces platelet-like particles. Leukemia. 2000;14:142–152. doi: 10.1038/sj.leu.2401608. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

