NGS-Guided Precision Oncology in Breast Cancer and Gynecological Tumors-A Retrospective Molecular Tumor Board Analysis
- PMID: 38672643
- PMCID: PMC11048446
- DOI: 10.3390/cancers16081561
NGS-Guided Precision Oncology in Breast Cancer and Gynecological Tumors-A Retrospective Molecular Tumor Board Analysis
Abstract
Background: Precision oncology treatments are being applied more commonly in breast and gynecological oncology through the implementation of Molecular Tumor Boards (MTBs), but real-world clinical outcome data remain limited. Methods: A retrospective analysis was conducted in patients with breast cancer (BC) and gynecological malignancies referred to our center's MTB from 2018 to 2023. The analysis covered patient characteristics, next-generation sequencing (NGS) results, MTB recommendations, therapy received, and clinical outcomes. Results: Sixty-three patients (77.8%) had metastatic disease, and forty-four patients (54.3%) had previously undergone three or more lines of systemic treatment. Personalized treatment recommendations were provided to 50 patients (63.3%), while 29 (36.7%) had no actionable target. Ultimately, 23 patients (29.1%) underwent molecular-matched treatment (MMT). Commonly altered genes in patients with pan-gyn tumors (BC and gynecological malignancies) included TP53 (n = 42/81, 51.9%), PIK3CA (n = 18/81, 22.2%), BRCA1/2 (n = 10/81, 12.3%), and ARID1A (n = 9/81, 11.1%). Patients treated with MMT showed significantly prolonged progression-free survival (median PFS 5.5 vs. 3.5 months, p = 0.0014). Of all patients who underwent molecular profiling, 13.6% experienced a major clinical benefit (PFSr ≥ 1.3 and PR/SD ≥ 6 months) through precision oncology. Conclusions: NGS-guided precision oncology demonstrated improved clinical outcomes in a subgroup of patients with gynecological and breast cancers.
Keywords: breast cancer; gynecological tumors; molecular tumor board; next-generation sequencing; precision oncology.
Conflict of interest statement
F.R.R. received speaker honoraria from AstraZeneca. C.D. received personal fees from Novartis, Roche, MSD Oncology, Daiichi Sankyo, AstraZeneca, Molecular Health, and Merck, all outside the submitted work. C.D. is cofounder of Sividon Diagnostics. In addition, C.D. has a patent on VMScope digital pathology software with royalties paid; a patent WO2020109570A1—cancer immunotherapy pending; and patents WO2015114146A1 and WO2010076322A1—therapy response issued. E.K.M.M. received speaker honoraria from Roche and Boehringer Ingelheim and served on an advisory board for Roche. The other authors declare no conflicts of interest.
Figures
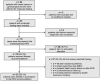
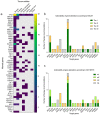
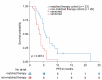
References
-
- Rosen E., Drilon A., Chakravarty D. Precision Oncology: 2022 in Review. Cancer Discov. 2022;12:2747–2753. doi: 10.1158/2159-8290.CD-22-1154. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

