Patients with Advanced or Metastasised Non-Small-Cell Lung Cancer with Viscum album L. Therapy in Addition to PD-1/PD-L1 Blockade: A Real-World Data Study
- PMID: 38672690
- PMCID: PMC11049173
- DOI: 10.3390/cancers16081609
Patients with Advanced or Metastasised Non-Small-Cell Lung Cancer with Viscum album L. Therapy in Addition to PD-1/PD-L1 Blockade: A Real-World Data Study
Abstract
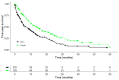
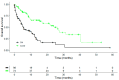
Immunotherapy with PD-1/PD-L1 inhibitors has significantly improved the survival rates of patients with metastatic non-small-cell lung cancer (NSCLC). Results of a real-world data study investigating add-on VA (Viscum album L.) to chemotherapy have shown an association with the improved overall survival of patients with NSCLC. We sought to investigate whether the addition of VA to PD-1/PD-L1 inhibitors in patients with advanced or metastasised NSCLC would have an additional survival benefit. In the present real-world data study, we enrolled patients from the accredited national registry, Network Oncology, with advanced or metastasised NSCLC. The reporting of data was performed in accordance with the ESMO-GROW criteria for the optimal reporting of oncological real-world evidence (RWE) studies. Overall survival was compared between patients receiving PD-1/PD-L1 inhibitor therapy (control, CTRL group) versus the combination of anti-PD-1/PD-L1 therapy and VA (combination, COMB group). An adjusted multivariate Cox proportional hazard analysis was performed to investigate variables associated with survival. From 31 July 2015 to 9 May 2023, 415 patients with a median age of 68 years and a male/female ratio of 1.2 were treated with anti-PD-1/PD-L1 therapy with or without add-on VA. Survival analyses included 222 (53.5%) patients within the CRTL group and 193 (46.5%) in the COMB group. Patients in the COMB group revealed a median survival of 13.8 months and patients in the CRTL group a median survival of 6.8 months (adjusted hazard ratio, aHR: 0.60, 95% CI: 0.43-0.85, p = 0.004) after adjustment for age, gender, tumour stage, BMI, ECOG status, oncological treatment, and PD-L1 tumour proportion score. A reduction in the adjusted hazard of death by 56% was seen with the addition of VA (aHR 0.44, 95% CI: 0.26-0.74, p = 0.002) in patients with PD-L1-positive tumours (tumour proportion score > 1%) treated with first-line anti-PD-1/PD-L1 therapy. Our findings suggest that add-on VA correlates with improved survival in patients with advanced or metastasised NSCLC who were treated with PD-1/PD-L1 inhibitors irrespective of age, gender, tumour stage, or oncological treatment. The underlying mechanisms may include the synergistic modulation of the immune response. A limitation of this study is the observational non-randomised study design, which only allows limited conclusions to be drawn and prospective randomised trials are warranted.
Keywords: PD-1 inhibitor; PD-L1 inhibitor; Viscum album L. extracts; lung cancer; non-small-cell lung cancer; real-world data study; survival.
Conflict of interest statement
C.G. reports honoraria from AstraZeneca, Novartis, Chiesi, the German Society of Pneumology, Takeda, the German S3-Guideline on Complementary Medicine in the Treatment of Oncological Patients, the Brandenburgian Cancer Society, and outside the submitted work as well as grants from Wala AG, Iscador AG, and outside the submitted work. C.G. is a member of the European Respiratory Society, the German Society of Pneumology, the Health Care without Harm, the German Alliance for Climate Change and Health, and the Society of Anthroposophic Physicians. F.S. reports grants from Helixor Heilmittel GmbH, Iscador AG, Abnoba GmbH, and outside the submitted work. H.M. has an endowed professorship at the Charité-University Hospital Berlin in Integrative and Anthroposophical Medicine, is a member of the board of directors at Weleda AG, a member of the board of the Hufelandgesellschaft e. V., and the president of the German Academy for Homeopathy and Naturopathy. R.-D.H. reports a consulting or advisory role for Amgen, Roche, Merck, Sanofi, Bayer, Ipsen, BMS, and MSD; honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer, Ipsen, Lilly, Medac, Merck, MSD, Roche, Saladax, Sanofi, and outside the submitted work; and research grants from Amgen, Medac, Merck, Roche, Saladax, Sanofi, and outside the submitted work. The other authors declare that they have no competing interests.
Figures

Similar articles
-
Immune Checkpoint Blockade Combined with AbnobaViscum® Therapy Is Linked to Improved Survival in Advanced or Metastatic Non-Small-Cell Lung Cancer Patients: A Registry Study in Accordance with the ESMO Guidance for Reporting Real-World Evidence.Pharmaceuticals (Basel). 2024 Dec 18;17(12):1713. doi: 10.3390/ph17121713. Pharmaceuticals (Basel). 2024. PMID: 39770555 Free PMC article.
-
Combined Immune Checkpoint Blockade and Helixor® Therapy in Oncology: Real-World Tolerability and Subgroup Survival (ESMO GROW).Int J Mol Sci. 2025 Apr 12;26(8):3669. doi: 10.3390/ijms26083669. Int J Mol Sci. 2025. PMID: 40332249 Free PMC article.
-
Overall survival of stage IV non-small cell lung cancer patients treated with Viscum album L. in addition to chemotherapy, a real-world observational multicenter analysis.PLoS One. 2018 Aug 27;13(8):e0203058. doi: 10.1371/journal.pone.0203058. eCollection 2018. PLoS One. 2018. PMID: 30148853 Free PMC article.
-
Viscum album L. Therapy in Oncology: An Update on Current Evidence.Complement Med Res. 2022;29(4):362-368. doi: 10.1159/000524184. Epub 2022 Mar 24. Complement Med Res. 2022. PMID: 35325897 Review. English.
-
FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies.Semin Oncol. 2018 Aug;45(4):220-225. doi: 10.1053/j.seminoncol.2018.08.007. Epub 2018 Oct 31. Semin Oncol. 2018. PMID: 30391014 Review.
Cited by
-
Immune Checkpoint Blockade Combined with AbnobaViscum® Therapy Is Linked to Improved Survival in Advanced or Metastatic Non-Small-Cell Lung Cancer Patients: A Registry Study in Accordance with the ESMO Guidance for Reporting Real-World Evidence.Pharmaceuticals (Basel). 2024 Dec 18;17(12):1713. doi: 10.3390/ph17121713. Pharmaceuticals (Basel). 2024. PMID: 39770555 Free PMC article.
-
Combined Immune Checkpoint Blockade and Helixor® Therapy in Oncology: Real-World Tolerability and Subgroup Survival (ESMO GROW).Int J Mol Sci. 2025 Apr 12;26(8):3669. doi: 10.3390/ijms26083669. Int J Mol Sci. 2025. PMID: 40332249 Free PMC article.
References
-
- Cancer.net Statistics, Non-Small Cell Lung Cancer. [(accessed on 4 February 2024)]. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics.
-
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

