Microfluidics-Based Drying-Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions
- PMID: 38672743
- PMCID: PMC11050796
- DOI: 10.3390/life14040472
Microfluidics-Based Drying-Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions
Abstract
Drying-wetting cycles play a crucial role in the investigation of the origin of life as processes that both concentrate and induce the supramolecular assembly and polymerization of biomolecular building blocks, such as nucleotides and amino acids. Here, we test different microfluidic devices to study the dehydration-hydration cycles of the aqueous solutions of small molecules, and to observe, by optical microscopy, the insurgence of phase transitions driven by self-assembly, exploiting water pervaporation through polydimethylsiloxane (PDMS). As a testbed, we investigate solutions of the chromonic dye Sunset Yellow (SSY), which self-assembles into face-to-face columnar aggregates and produces nematic and columnar liquid crystal (LC) phases as a function of concentration. We show that the LC temperature-concentration phase diagram of SSY can be obtained with a fair agreement with previous reports, that droplet hydration-dehydration can be reversibly controlled and automated, and that the simultaneous incubation of samples with different final water contents, corresponding to different phases, can be implemented. These methods can be further extended to study the assembly of diverse prebiotically relevant small molecules and to characterize their phase transitions.
Keywords: chromonic liquid crystals; dry–wet cycles; microfluidics; self-assembly.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
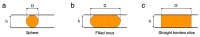

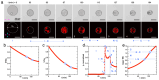

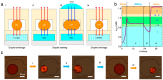


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

