Microstructure and Chlorine Ion Corrosion Performance in Bronze Earring Relics
- PMID: 38673090
- PMCID: PMC11051321
- DOI: 10.3390/ma17081734
Microstructure and Chlorine Ion Corrosion Performance in Bronze Earring Relics
Abstract
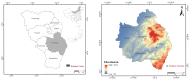
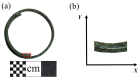
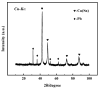
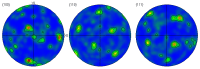
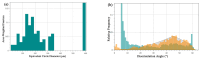
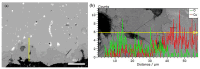
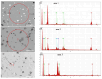
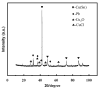
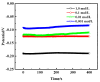
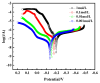
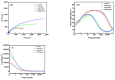
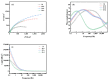
Chlorine ions play an important role in the corrosion of bronzeware. This study employs techniques such as XRD, OM, SEM, EBSD, and electrochemical testing to analyze the microstructure, crystal structure, chemical composition, and corrosion performance of bronze earrings unearthed at the Xindianzi site in Inner Mongolia. The results indicate the presence of work-hardened structures, including twinning and equiaxed crystals, on the earrings' surface. With an increase in chloride ion concentration in NaCl solutions from 10-3 mol/L to 1 mol/L, the corrosion current density of the bronze earrings increased from 2.372 × 10-7 A/cm2 to 9.051 × 10-7 A/cm2, demonstrating that the alloy's corrosion rate escalates with chloride ion concentration. A 3-day immersion test in 0.5% NaCl solution showed the formation of a passivation layer of metal oxides on the earrings' surface. These findings underscore the significance of the impact chloride ions have on the corrosion of copper alloys, suggesting that activating the alloy's reactive responses can accelerate the corrosion process and provide essential insights into the corrosion mechanisms of bronze artifacts in chloride-containing environments.
Keywords: NaCl solution; alloy structure; bronze earrings; electrochemical corrosion; polarization curve.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Comparative Study of the Corrosive Behaviors of Rust Layers on Bronze Ware in Different Corrosive Environments.Materials (Basel). 2025 Mar 19;18(6):1359. doi: 10.3390/ma18061359. Materials (Basel). 2025. PMID: 40141649 Free PMC article.
-
The Corrosion Properties of Bronze Alloys in NaCl Solutions.Materials (Basel). 2023 Jul 21;16(14):5144. doi: 10.3390/ma16145144. Materials (Basel). 2023. PMID: 37512417 Free PMC article.
-
Alloy Microstructure Dictates Corrosion Modes in THA Modular Junctions.Clin Orthop Relat Res. 2017 Dec;475(12):3026-3043. doi: 10.1007/s11999-017-5486-3. Epub 2017 Sep 7. Clin Orthop Relat Res. 2017. PMID: 28884275 Free PMC article.
-
Synergistic effect of Mg addition on the enhancement of the mechanical properties and evaluation of corrosion behaviors in 3.5 wt % NaCl of aluminum alloys.Heliyon. 2024 Jan 28;10(3):e25437. doi: 10.1016/j.heliyon.2024.e25437. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38327413 Free PMC article.
-
Effects of Strontium Modification on Corrosion Resistance of Al-Si Alloys in Various Corrosive Environments.Materials (Basel). 2024 Oct 9;17(19):4923. doi: 10.3390/ma17194923. Materials (Basel). 2024. PMID: 39410493 Free PMC article.
Cited by
-
Comparative Study of the Corrosive Behaviors of Rust Layers on Bronze Ware in Different Corrosive Environments.Materials (Basel). 2025 Mar 19;18(6):1359. doi: 10.3390/ma18061359. Materials (Basel). 2025. PMID: 40141649 Free PMC article.
References
-
- Wu Z., Zhu Z., Wu R. Thermodynamic analysis of nanocrystalline solid solutions formation in copper-lead-tin ternary immiscible system during mechanical alloying. Mater. Werkst. 2021;52:1328–1337. doi: 10.1002/mawe.202100076. - DOI
-
- Chang T., Herting G., Goidanich S., Amaya J.S., Arenas M., Le Bozec N., Jin Y., Leygraf C., Wallinder I.O. The role of Sn on the long-term atmospheric corrosion of binary Cu-Sn bronze alloys in architecture. Corros. Sci. 2019;149:54–67. doi: 10.1016/j.corsci.2019.01.002. - DOI
-
- Dong B., Jie J., Peng B., Qu J., Wu Z., Wang Q., Zhou J., Liu S., Zou Q., Wang T. Revealing the Relationship between Morphology of Pb-Rich Secondary Phases and Mechanical Properties of Laminated Cu–Pb–Sn/Steel Composite Through CALPHAD and FEA. Metall. Mater. Trans. A. 2022;53:1462–1478. doi: 10.1007/s11661-022-06609-1. - DOI
-
- Cao J., Sun J. 21st-Century Archaeological Discoveries of the Early Nomadic Cultural Remains–Centered in the Middle Section of the Great Wall Area in Inner Mongolia Autonomous Region. J. Sib. Fed. Univ. 2021;14:1121–1138.
-
- Han H., Bai W., Liu D., Han J. Comprehensive Research on the Layered Corrosion of Archaeological Copper Wares: A Case Study from Qinghai, China. Stud. Conserv. 2023:1–9.
LinkOut - more resources
Full Text Sources

