The Solvent Role for the Decomposition of Paracetamol in Distilled and Drinking Water by Pure and Ag-Modified TiO2 Sol-Gel Powders
- PMID: 38673148
- PMCID: PMC11051041
- DOI: 10.3390/ma17081791
The Solvent Role for the Decomposition of Paracetamol in Distilled and Drinking Water by Pure and Ag-Modified TiO2 Sol-Gel Powders
Abstract
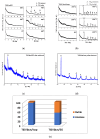
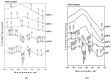
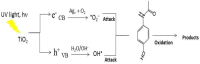
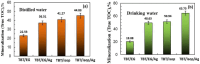
In this study, pure TiO2 gels were synthesized by applying the sol-gel method, using Ti(IV) butoxide with the addition of two different solvents, namely ethylene glycol (EG) and isopropanol (isop), with only air moisture present. It was established using XRD that the gel prepared with the addition of EG was amorphous even at 400 °C, while the other gel was amorphous up to 300 °C. It was found that TiO2 (anatase) had a dominant crystalline phase during heating to 600 °C, while at 700 °C, TiO2 (rutile) appeared. The as-obtained powdered materials were annealed at 500 °C and subsequently underwent photocatalytic tests with paracetamol. Additionally, the TiO2 samples were modified with Ag+ co-catalysts (10-2 M), using photofixation by UV illumination. The photocatalytic activity of the Ag-modified powders was also tested in the photodegradation of a commonly used paracetamol in aqueous solution under UV light illumination. The obtained data exhibited that the annealed samples had better photocatalytic efficiency and decomposed paracetamol faster in comparison to the non-annealed sol-gel powders. The highest degradation efficiency was observed for the TBT/isop/Ag material, with degradation efficiencies average values of 65.59% and 75.61% paracetamol achieved after the third cycle of photocatalytic treatment. The co-catalytically modified powders had higher photocatalytic efficiency in comparison to the pure nanosized powders. Moreover, the sol-gel powders of TBT/EG, TBT/EG/Ag (10-2 M), TBT/isop, and TBT/isop/Ag (10-2 M) demonstrated the ability to retain their photocatalytic activity even after three cycles of use, suggesting that they could find practical use in the treatment of pharmaceutical wastewater. The observed photocatalytic efficiency and positive impact of silver make the prepared powders a desirable choice for pharmaceutical drug degradation, helping to promote environmentally friendly and effective wastewater treatment technology.
Keywords: Ag/TiO2 powders; distilled and drinking water; paracetamol; photocatalytic degradation; sol–gel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Synthesis, Luminescent and Antibacterial Properties of Sol-Gel TiO2/TeO2/Nb2O5 Powders.Materials (Basel). 2025 Feb 21;18(5):946. doi: 10.3390/ma18050946. Materials (Basel). 2025. PMID: 40077173 Free PMC article.
-
Photocatalytic and Antibacterial Properties of Doped TiO2 Nanopowders Synthesized by Sol-Gel Method.Gels. 2022 Oct 20;8(10):673. doi: 10.3390/gels8100673. Gels. 2022. PMID: 36286174 Free PMC article.
-
UV-visible light-activated Ag-decorated, monodisperse TiO2 aggregates for treatment of the pharmaceutical oxytetracycline.Environ Sci Pollut Res Int. 2014 Oct;21(20):11781-93. doi: 10.1007/s11356-013-2233-5. Epub 2013 Nov 12. Environ Sci Pollut Res Int. 2014. PMID: 24217967
-
Three-Phase Mixed Titania Powder Modified by Silver and Silver Chloride with Enhanced Photocatalytic Activity under UV-Visible Light.Nanomaterials (Basel). 2022 May 9;12(9):1599. doi: 10.3390/nano12091599. Nanomaterials (Basel). 2022. PMID: 35564308 Free PMC article.
-
Photocatalytic Degradation of Paracetamol in Aqueous Medium Using TiO2 Prepared by the Sol-Gel Method.Molecules. 2022 May 2;27(9):2904. doi: 10.3390/molecules27092904. Molecules. 2022. PMID: 35566255 Free PMC article.
Cited by
-
Interface Effects on the Electronic and Optical Properties of Graphitic Carbon Nitride (g-C3N4)/SnS2: First-Principles Studies.Materials (Basel). 2025 Feb 18;18(4):892. doi: 10.3390/ma18040892. Materials (Basel). 2025. PMID: 40004415 Free PMC article.
References
-
- Bokov D., Abduladheem T.J., Chupradit S., Suksatan W., Ansari M.J., Shewael I.H., Gabdrakhman H. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021;2021:5102014. doi: 10.1155/2021/5102014. - DOI
-
- Gupta S., Tripathi M. A review on the synthesis of TiO2 nanoparticles by solution route. Open Chem. 2012;10:279–294. doi: 10.2478/s11532-011-0155-y. - DOI
-
- Niederberger M., Pinna N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application. Springer; New York, NY, USA: 2009.
-
- Collinson M.M., Wang H., Makote R., Khramov A. The effects of drying time and relative humidity on the stability of sol-gel derived silicate films in solution. J. Electroanal. Chem. 2002;519:65–71. doi: 10.1016/S0022-0728(01)00723-9. - DOI
LinkOut - more resources
Full Text Sources

